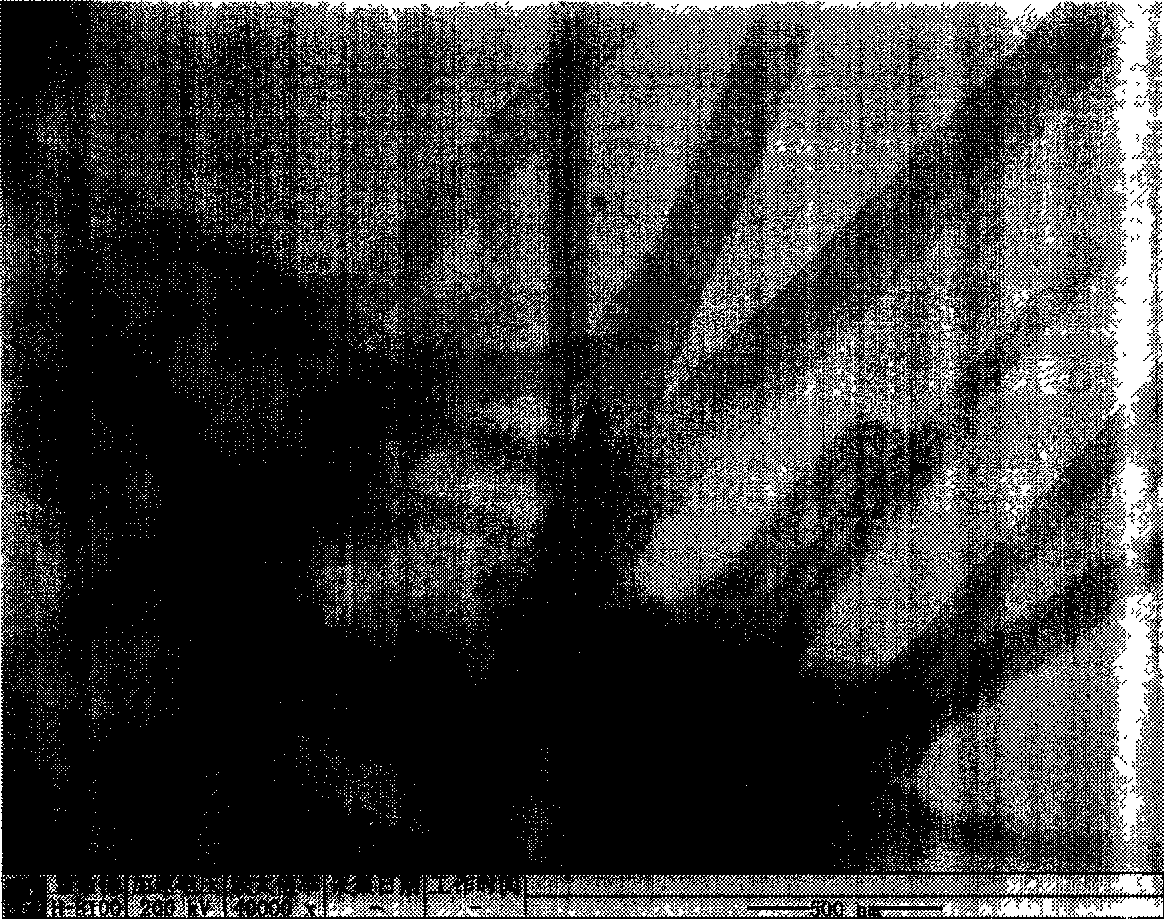Method for producing zinc hydroxide nano-wire
A technology of zinc hydroxide and nanowires, applied in the direction of zinc oxide/zinc hydroxide, etc., to achieve good stability, low cost, and overcome cost and environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Weigh 2.195g Zn(Ac) 2 2H 2 O, added to 100ml of absolute ethanol, and refluxed at 80°C until the solid dissolved. Add 0.842g KOH to 100ml of absolute ethanol, and dissolve it by ultrasonication. After the zinc acetate alcoholic solution returns to room temperature and the solution is clear, the potassium hydroxide alcoholic solution and the zinc acetate alcoholic solution are mixed. The above mixed solution was ultrasonicated for 1 hour to obtain a colorless and transparent zinc oxide sol. Slowly drip 10ml of water into 20ml of zinc oxide sol, and after standing for 1 day at room temperature, the layered zinc hydroxide acetate is obtained, and its morphology is analyzed with a transmission electron microscope (TEM). figure 1 . The obtained precipitate was centrifuged and washed three times with alcohol, then centrifuged and washed twice with deionized water, then the supernatant was discarded, and placed in 4 ml of water for 3 days to obtain zinc hydroxide nanowires...
Embodiment 2
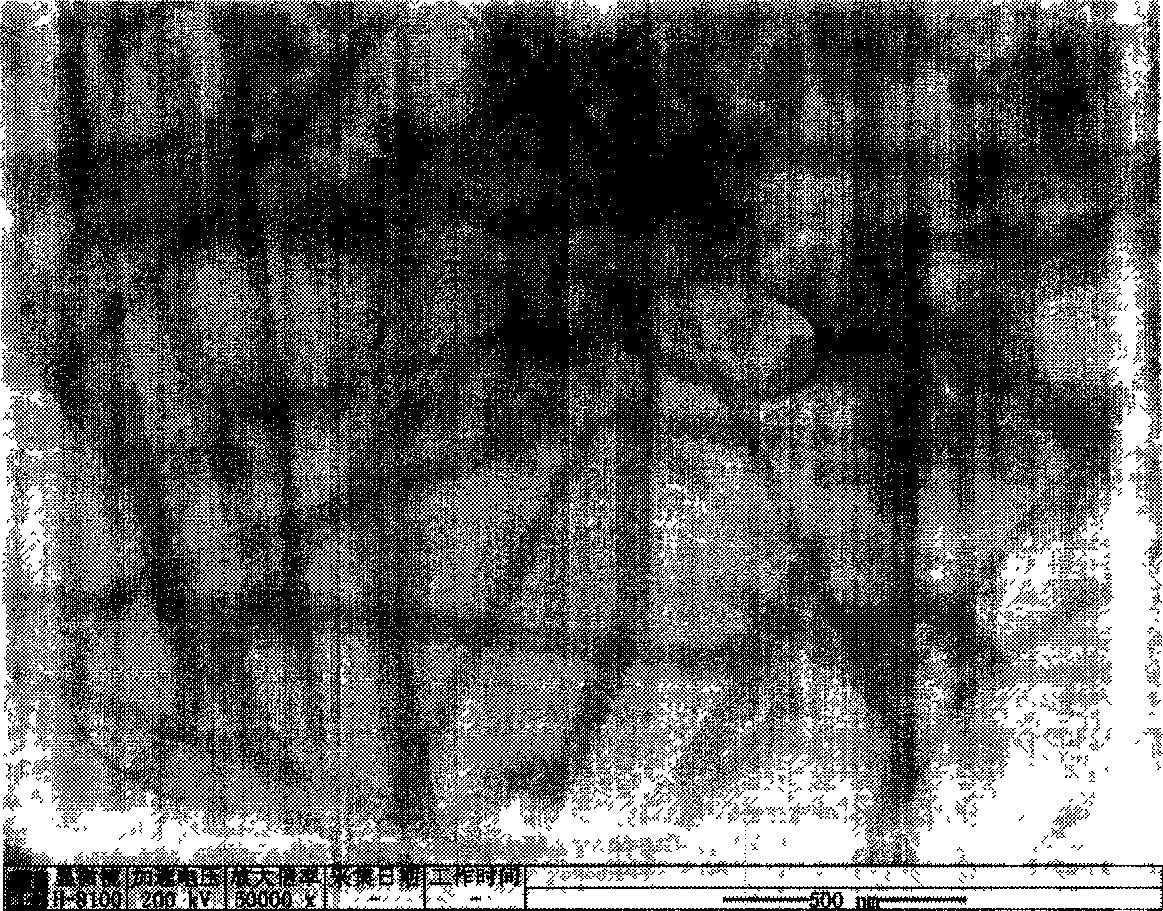
[0023] Weigh 2.195g Zn(Ac) 2 2H 2 O, add to 100ml of absolute ethanol, reflux at 78°C until the solid dissolves. Add 0.842g KOH to 100ml of absolute ethanol, and dissolve it by ultrasonication. After the zinc acetate alcoholic solution returns to room temperature and the solution is clear, the potassium hydroxide alcoholic solution and the zinc acetate alcoholic solution are mixed. The above mixed solution was ultrasonicated for 2 hours to obtain a colorless and transparent zinc oxide sol. 10ml of water was slowly dropped into 20ml of zinc oxide sol, and after standing at room temperature for 1 day, layered zinc hydroxide acetate was obtained. The obtained precipitate was centrifuged and washed three times with alcohol, then centrifuged and washed twice with deionized water, then the supernatant was discarded, and placed in 4 ml of water for 1 day to obtain zinc hydroxide nanowires. It was analyzed with a transmission electron microscope (TEM), and its morphology was obser...
Embodiment 3
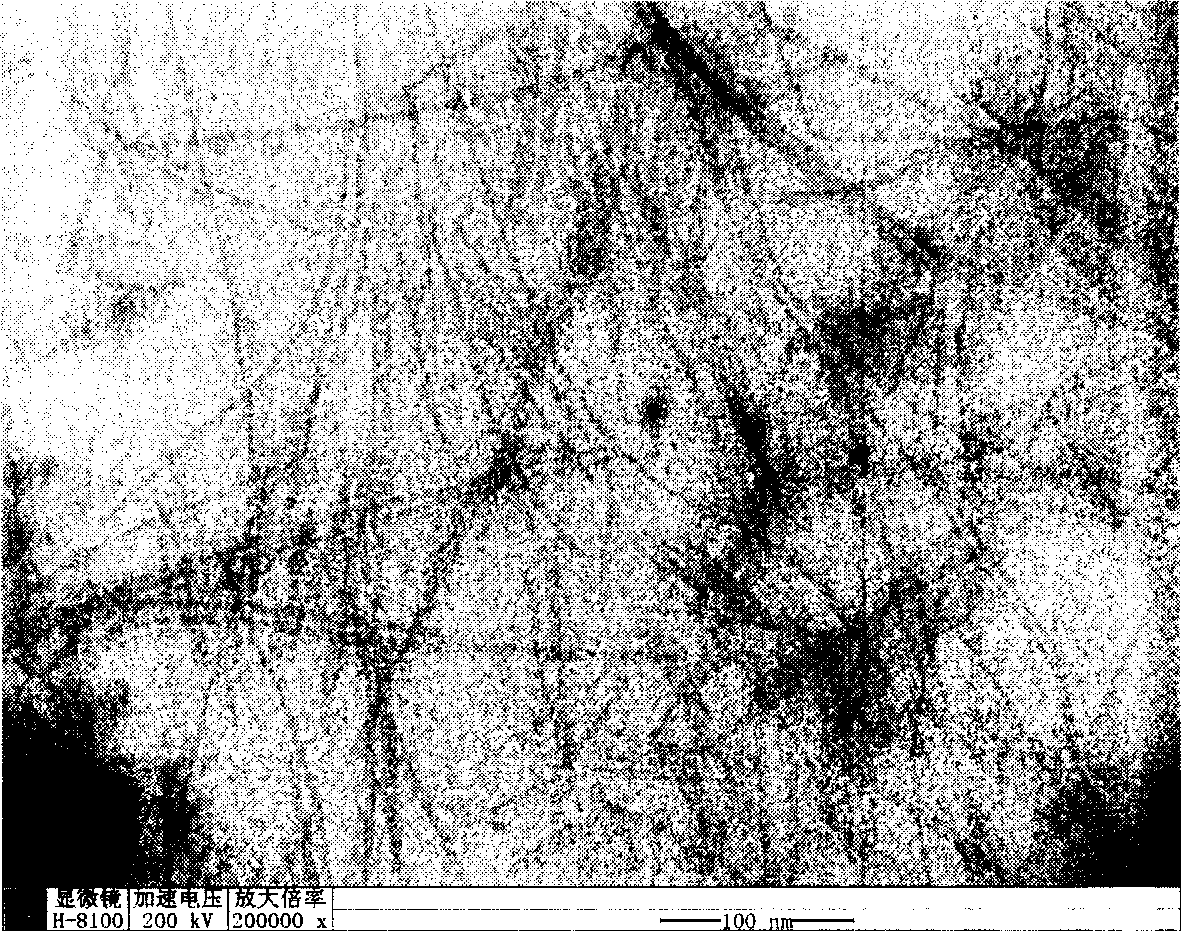
[0025] Weigh 2.195g Zn(Ac) 2 2H 2 O, added to 100ml of absolute ethanol, and refluxed at 90°C until the solid dissolved. Add 0.842g KOH to 100ml of absolute ethanol, and dissolve it by ultrasonication. After the zinc acetate alcoholic solution returns to room temperature (the solution must be clear), mix the potassium hydroxide alcoholic solution and the zinc acetate alcoholic solution. The above mixed solution was ultrasonicated for 1 hour to obtain a colorless and transparent zinc oxide sol. 10ml of water was slowly dropped into 20ml of zinc oxide sol, and after standing at room temperature for one day, layered zinc hydroxide acetate was obtained. The obtained precipitate was centrifuged and washed 4 times with alcohol, centrifuged and deionized water washed 2 times, then the supernatant was discarded, and placed in 3 ml of water for 4 days to obtain zinc hydroxide nanowires. It was analyzed with a transmission electron microscope (TEM), and its morphology was observed t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com