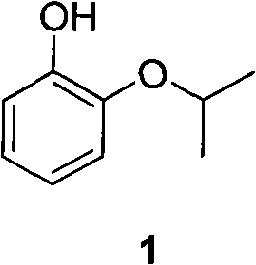
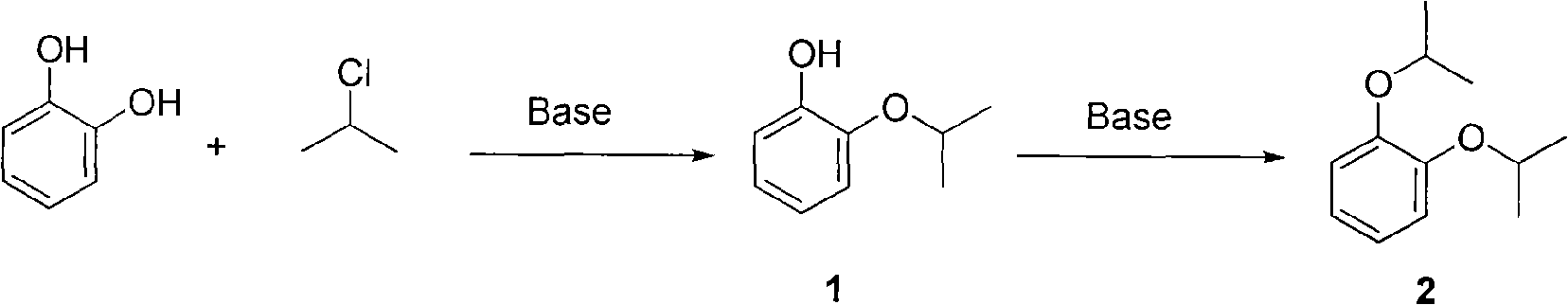
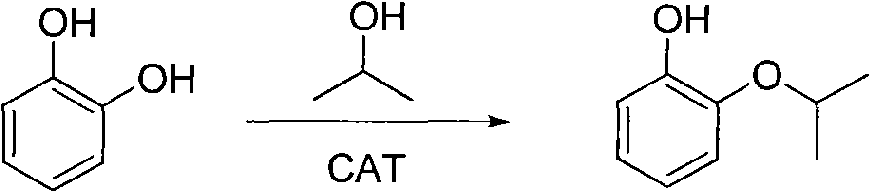
Preparation method of o-isopropoxyphenol
A technology of propoxyphenol and catechol, which is applied in the field of preparation of o-isopropoxyphenol, can solve the problems of no industrial value, high cost of raw materials, serious pollution, etc., and achieve remarkable benefits of energy saving, emission reduction and environmental protection , cheap raw materials, high yield effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Add 22g (0.2mol) catechol, 16g (0.11mol) potassium carbonate, 100mL ethylene glycol, 200mL petroleum ether, catalyst tetrabutylammonium bromide 0.2g into a 1000mL autoclave, stir and heat up to 90°C, and 24g (0.3mol) 2-chloropropane was added in 4h, and the reaction was continued for 4h after the addition was completed. After the reaction was completed, the temperature was lowered, and the reaction liquid was filtered, and the filtrate was separated after standing. (the area of the liquid chromatography is normalized), the content of pyrocatechol is 2.0% (the area of the liquid chromatography is normalized), after reclaiming the solvent, rectification under negative pressure obtains o-isopropoxyphenol; the lower phase is a polar solvent phase , the content of catechol is 88.0% (normalized by the area of liquid chromatography), and the content of monoether is 10.0% (normalized by the area of liquid chromatography), which can be directly recycled as mother liquor. ...
Embodiment 2
[0026] Add 22g (0.2mol) catechol, 14g (0.11mol) sodium carbonate, 100mL glycerol, 200mL n-octane, catalyst tetramethylammonium bromide 0.3g into a 1000mL autoclave, stir and heat up to 110°C, and 24g (0.3mol) 2-chloropropane was added in 4 hours, and continued to react for 2 hours after the addition was completed. After the reaction was completed, the temperature was lowered, and the reaction liquid was filtered, and the filtrate was separated after standing. % (normalized liquid chromatography area), the catechol content is 2.9% (normalized liquid chromatography area), after reclaiming the solvent, vacuum rectification obtains o-isopropoxyphenol; the lower phase is middle catechol The content of phenol is 85.6% (normalized by the area of liquid chromatography), and the content of monoether is 10.5% (normalized by the area of liquid chromatography), which are directly used as mother liquor for recycling. The monoether selectivity of the reaction was 98.5%.
[0027] The mo...
Embodiment 3
[0029] Add 22g (0.2mol) catechol, 12g (0.11mol) ammonium carbonate, 150mL diethylene glycol, 300mL n-nonane, catalyst tetrabutylammonium bromide 0.4g into a 1000mL autoclave, stir and heat up to 130°C, and 24g (0.3mol) 2-chloropropane was added in 4 hours, and continued to react for 1 hour after the addition was completed. After the reaction was completed, the temperature was lowered, and the reaction liquid was filtered, and the filtrate was separated after standing. % (normalized by liquid chromatography area), the catechol content is 4.1% (normalized by liquid chromatography area), after reclaiming the solvent, vacuum distillation obtains o-isopropoxyphenol; in the lower phase, catechol The content is 86.2% (normalized by the area of liquid chromatography), and the content of monoether is 12.2% (normalized by the area of liquid chromatography), which is directly used as mother liquor for recycling. The monoether selectivity of the reaction was 99.3%.
[0030] The mothe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com