Processes for preparing cinacalcet hydrochloride and polymorphic forms thereof
A technology of cinacalcet hydrochloride and cinacalcet, which is applied in the field of cinacalcet hydrochloride, can solve the problems such as no specific examples of preparation of cinacalcet hydrochloride reported
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0204] Example 1: Preparation of Cinacalcet Hydrochloride
[0205] In an argon atmosphere, 1.69 g (9.89 mmol, 1.1 equiv) of (R)-1-naphthylethylamine was added to 2.0 g (8.93 mmol, GC purity: 90.3%) of 3-(3-trifluoromethane) phenyl)propanal in 40 mL of tetrahydrofuran. The resulting clear solution was stirred for 15 minutes, 2 mL of acetic acid and 3.18 g (15.0 mmol) of sodium triacetoxyborohydride were added. The reaction mixture was stirred for 2 hours and the solvent was evaporated in vacuo. The resulting residue was dissolved in 30 mL of dichloromethane, and the resulting solution was washed with 30 mL of 10% sodium carbonate solution. The inorganic layer was extracted with 20 mL of dichloromethane and the solvent of the collected organic phase was evaporated in vacuo. The resulting crude base (3.17 g, 89%) was then dissolved in 5 mL of ethyl acetate and acidified with hydrochloric acid in diethyl ether. The evaporated crude salt was then treated with 2-3 mL of ethyl ac...
Embodiment 2
[0207] Example 2: Preparation of Cinacalcet Hydrochloride
[0208] To a cold (10°C) solution of 19.25 g (112 mmol) of (R)-1-naphthylethylamine was alternately added 4.5 mL of acetic acid and 500 mL of isobutyl acetate, 150 mL of freshly prepared 8 portions over 4 hours of sodium triacetoxyborohydride and 25.0 g (124.0 mmol, 96.7%) of 3-(3-trifluoromethylphenyl)propanal in 100 mL of isobutyl acetate, starting from the reducing agent. An aliquot of the borohydride was added simultaneously, while an aliquot of the aldehyde was added dropwise over 10 minutes. After the addition was complete, the resulting white suspension was stirred for 20 minutes, and then 300 mL of distilled water was added. Next, 100 mL of a 10% aqueous sodium carbonate solution was added dropwise. The organic layer was separated and concentrated to about 250 mL. To the concentrated solution was added 75 mL of 2M aqueous hydrochloric acid followed by 150 mL of heptane with stirring. The precipitated crude ...
Embodiment 3
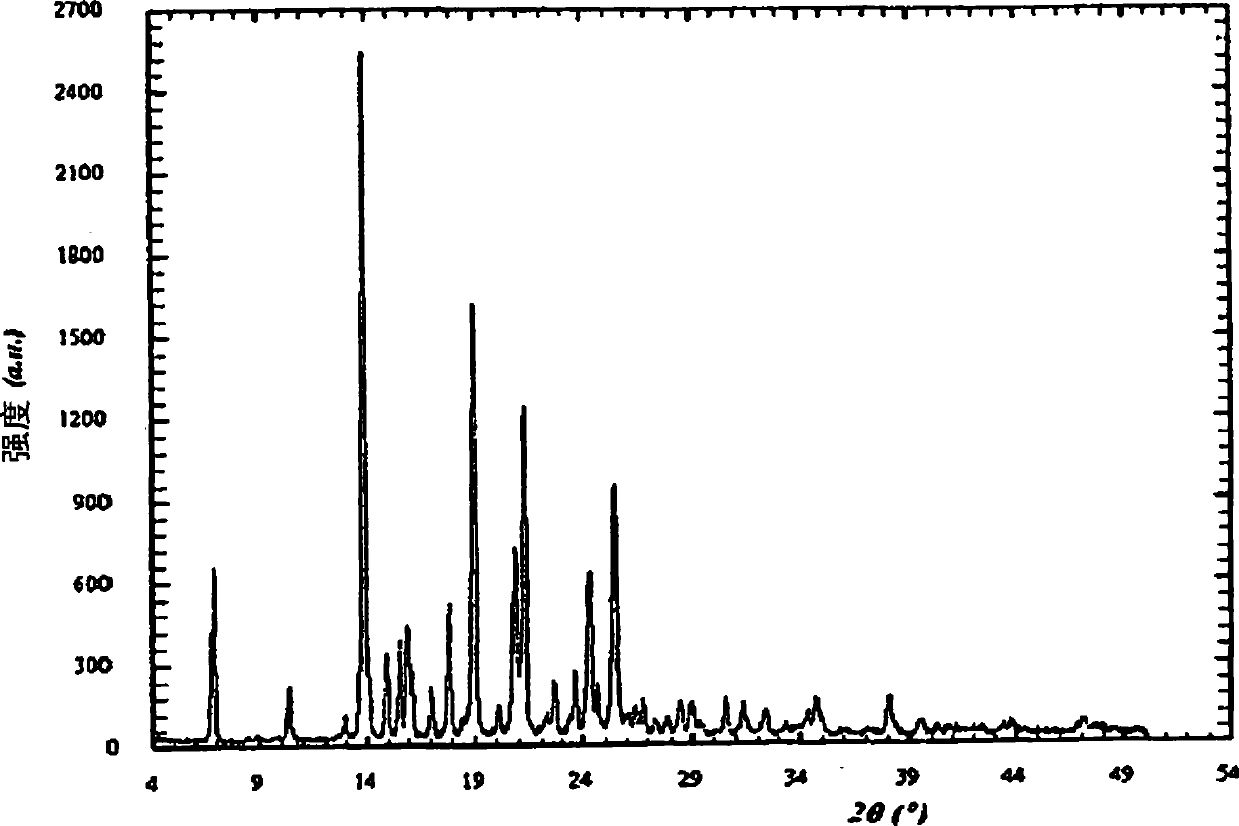
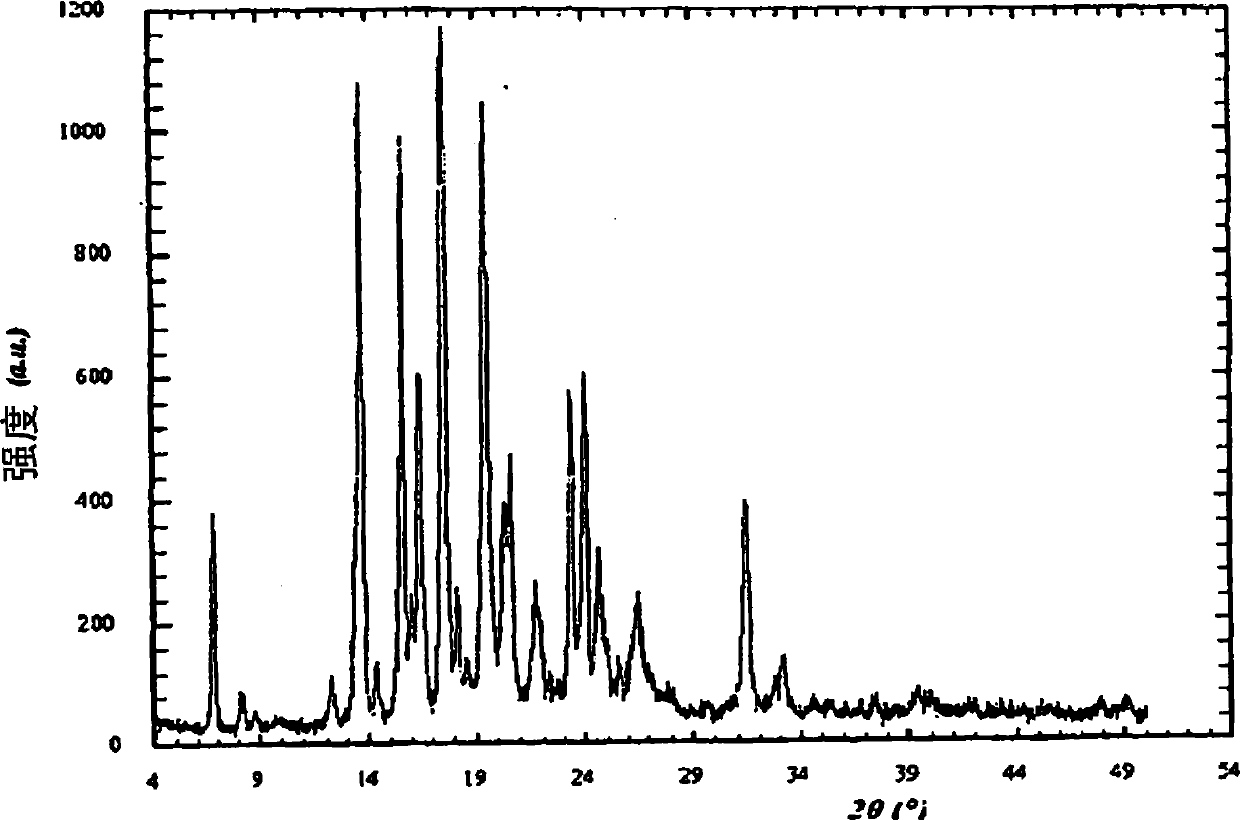
[0210] Example 3: General procedure for the preparation of Cinacalcet hydrochloride salt form I by evaporation
[0211] Solutions of cinacalcet hydrochloride were obtained in appropriate solvents at the concentrations shown in Table 1. This solution was slowly evaporated at room temperature and the resulting solid was ground smoothly for XRD analysis. The results are summarized in Table 1.
[0212] solvent Concentration (Volume) XRD acetone 25 Form 1 Ethanol 5 Form 1 2-Propanol 35 Form 1 methyl ethyl ketone 25 Form 1 Dichloromethane 3 Form 1 Ethyl acetate 80 Form 1 2-Butanol 60 Form 1 2-Methyltetrahydrofuran 50 Form 1 dimethylformamide 5 Form 1 dimethylacetamide 5 Form 1 dimethyl sulfoxide 5 Form 1 1,4-Dioxane 23 Form 1
[0213] Table 1
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com