Method for synthesizing cinacalcet intermediate
A technology of cinacalcet and an intermediate is applied in the field of preparation of cinacalcet hydrochloride intermediate-N-ethyl-3-phenyl) propionamide, and can solve the problems of difficult to scale up production, high cost, difficult separation and the like, To achieve the effect of mild reaction temperature, low equipment requirements and high economic value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
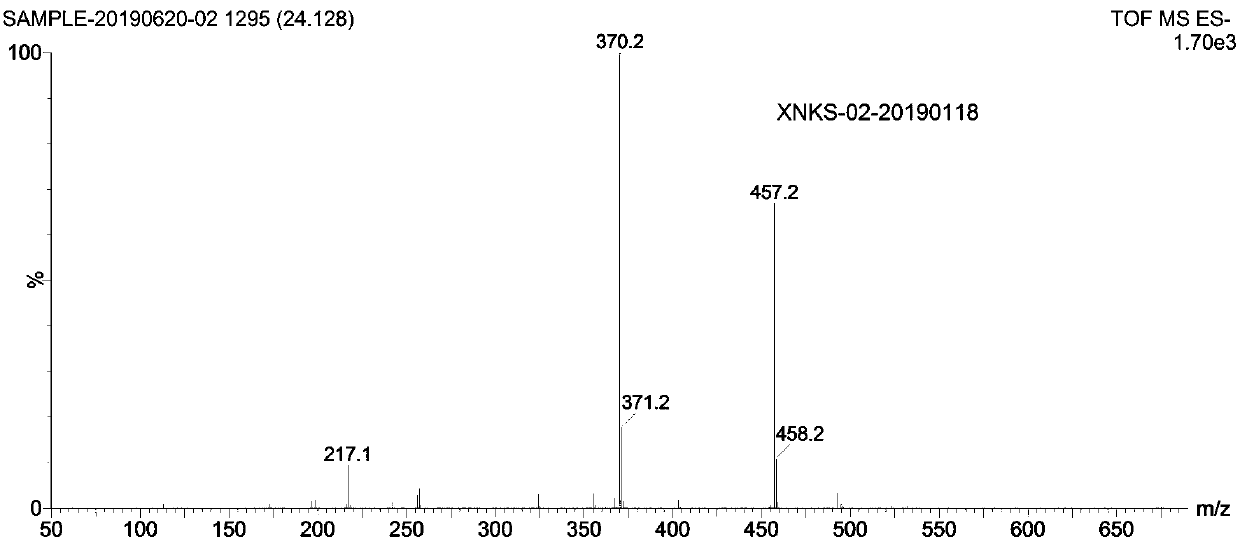
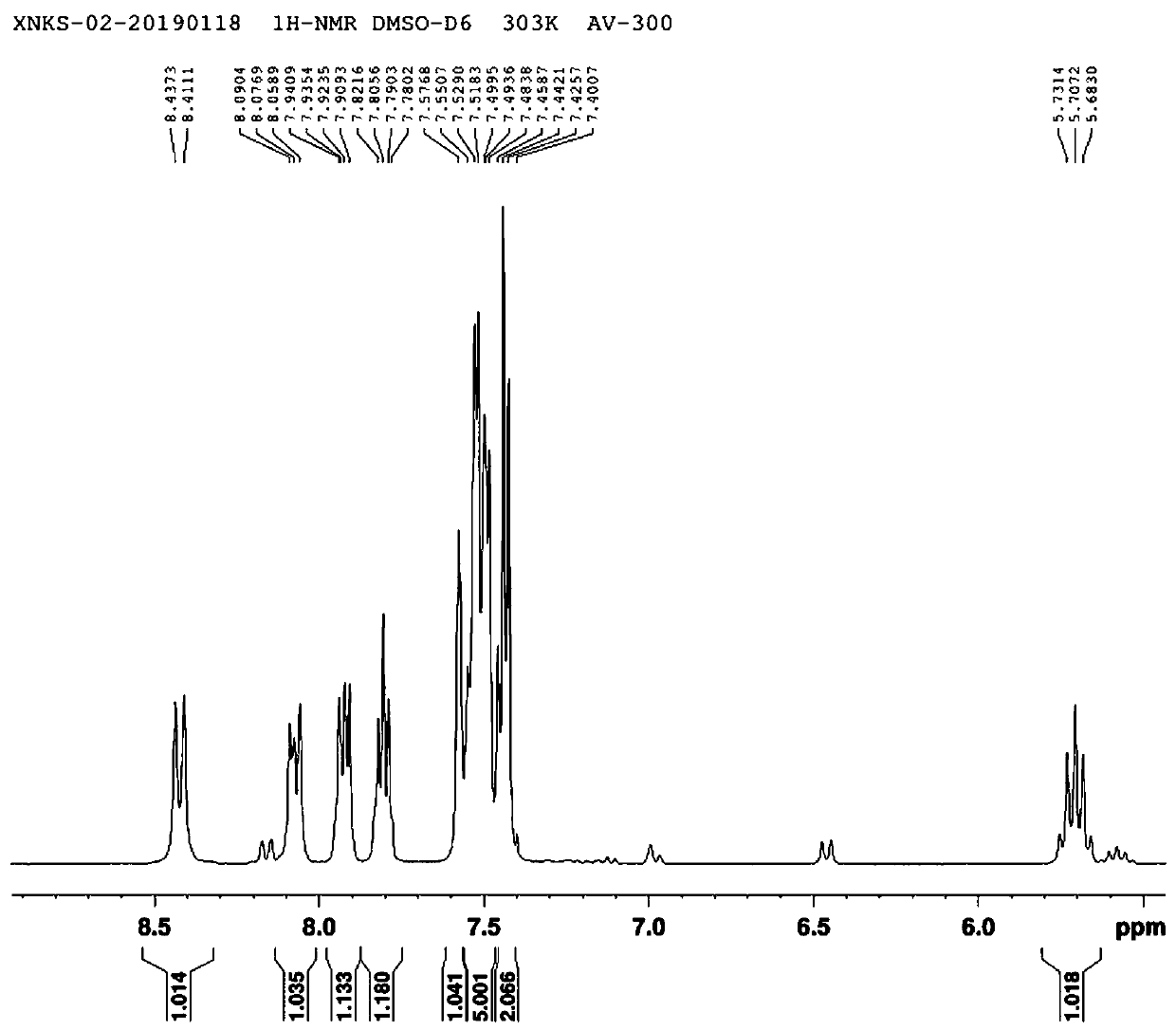
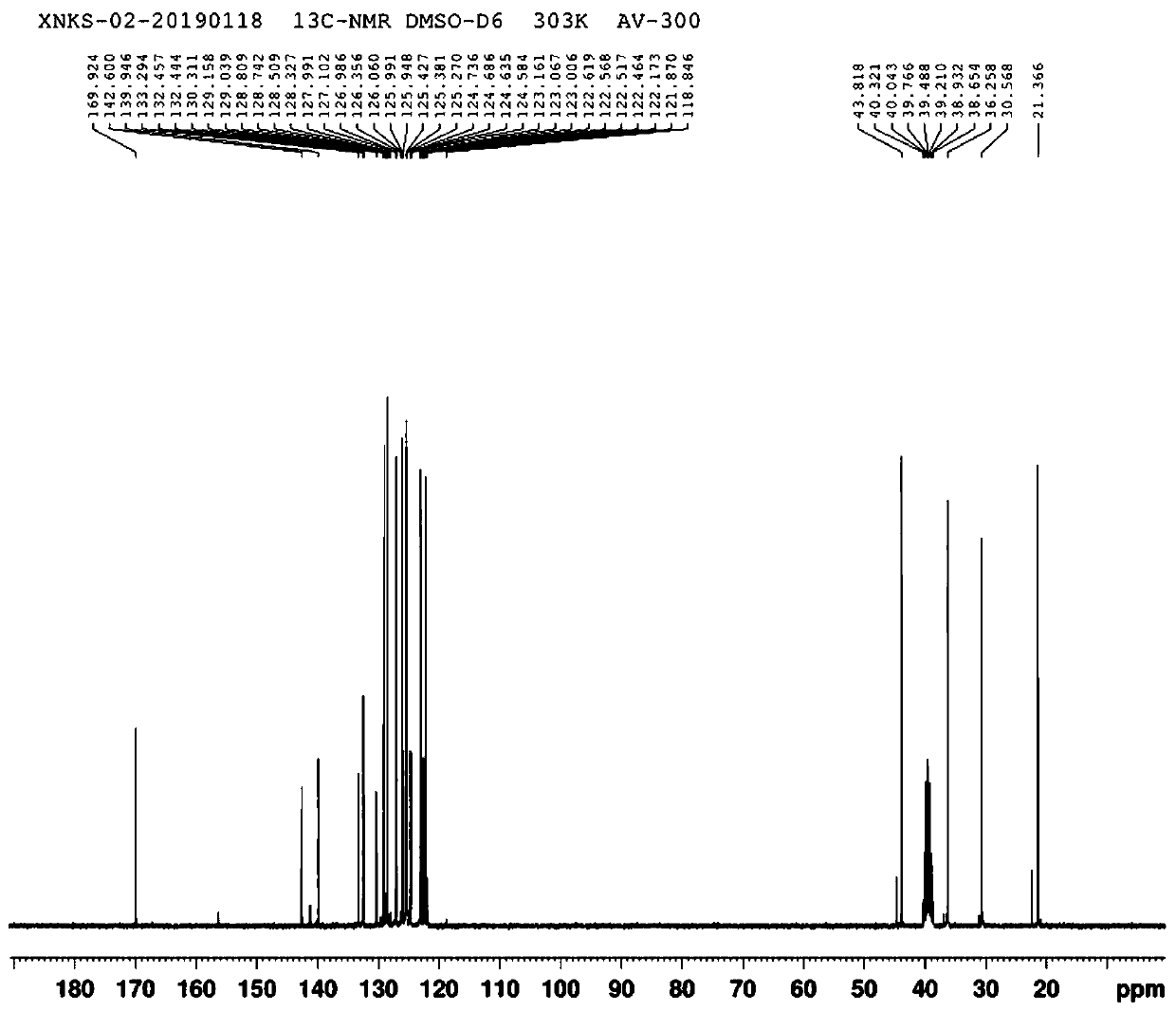
[0035] Add 10g of "3-(3-trifluoromethylphenyl)propionic acid" to a 250ml three-necked flask, add 60ml of toluene, protect with nitrogen, add 6g of thionyl chloride dropwise, and add 50mg of DMF as a catalyst. After the addition, The temperature was raised to 47±3°C for 2 hours, and the reaction system was decompressed and rotated to remove toluene and excess thionyl chloride to obtain 11 g of "3-(3-trifluoromethylphenyl)propionyl chloride". The obtained "3-( 3-Trifluoromethylphenyl) propionyl chloride" was dissolved in 45ml of acetonitrile in a 250ml three-necked flask, and 7.8g of "(R)-1-(1-naphthyl)ethylamine" and 6.4g of anhydrous potassium carbonate were added, and the addition was completed After that, the temperature was raised to reflux for 22 hours, the reaction system was filtered, and the filtrate was distilled to dryness under reduced pressure to obtain 18 g of solid. The solid was dissolved in 130ml of toluene, and 37ml of 32% hydrochloric acid was added to adjust ...
Embodiment 2
[0038] Add 10g of "3-(3-trifluoromethylphenyl)propionic acid" to a 250ml three-necked flask, add 60ml of toluene, protect with nitrogen, add 6g of thionyl chloride dropwise, and add 50mg of DMF as a catalyst. After the addition, The temperature was raised to 48±3°C for 2 hours, and the reaction system was decompressed and rotated to remove toluene and excess thionyl chloride to obtain 11 g of "3-(3-trifluoromethylphenyl)propionyl chloride". The obtained "3-( 3-Trifluoromethylphenyl) propionyl chloride" was dissolved in 45ml of acetonitrile in a 250ml three-necked flask, and 7.8g of "(R)-1-(1-naphthyl)ethylamine" and 6.4g of anhydrous potassium carbonate were added, and the addition was completed After that, the temperature was raised to reflux for 22 hours, the reaction system was filtered, and the filtrate was distilled to dryness under reduced pressure to obtain 18 g of solid. The solid was dissolved in 130ml of toluene, and 37ml of 32% hydrochloric acid was added to adjust ...
Embodiment 3
[0041] Add 10g of "3-(3-trifluoromethylphenyl)propionic acid" to a 250ml three-necked flask, add 60ml of toluene, protect with nitrogen, add 6g of thionyl chloride dropwise, and add 50mg of DMF as a catalyst. After the addition, Raise the temperature to 46±3°C and react for 2 hours. The reaction system was decompressed and rotated to remove toluene and excess thionyl chloride to obtain 11 g of "3-(3-trifluoromethylphenyl)propionyl chloride". The obtained "3-( 3-Trifluoromethylphenyl) propionyl chloride" was dissolved in 45ml of acetonitrile in a 250ml three-necked flask, and 7.8g of "(R)-1-(1-naphthyl)ethylamine" and 6.4g of anhydrous potassium carbonate were added, and the addition was completed After that, the temperature was raised to reflux for 22 hours, the reaction system was filtered, and the filtrate was distilled to dryness under reduced pressure to obtain 18 g of solid. The solid was dissolved in 130ml of toluene, and 37ml of 32% hydrochloric acid was added to adjust...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com