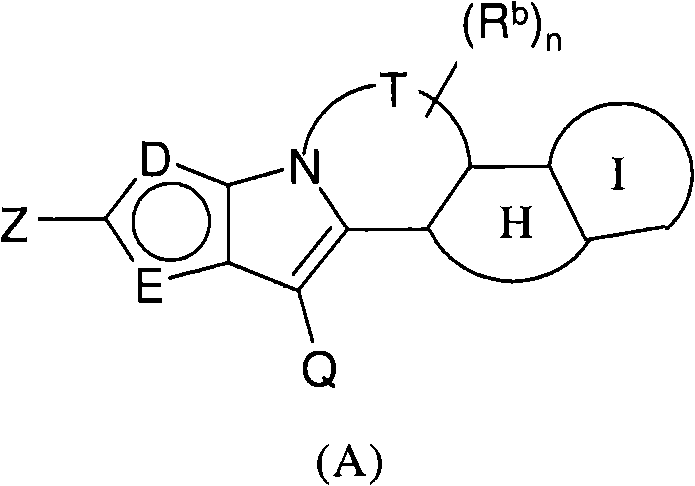
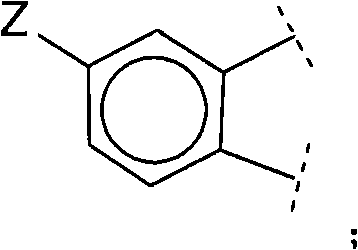
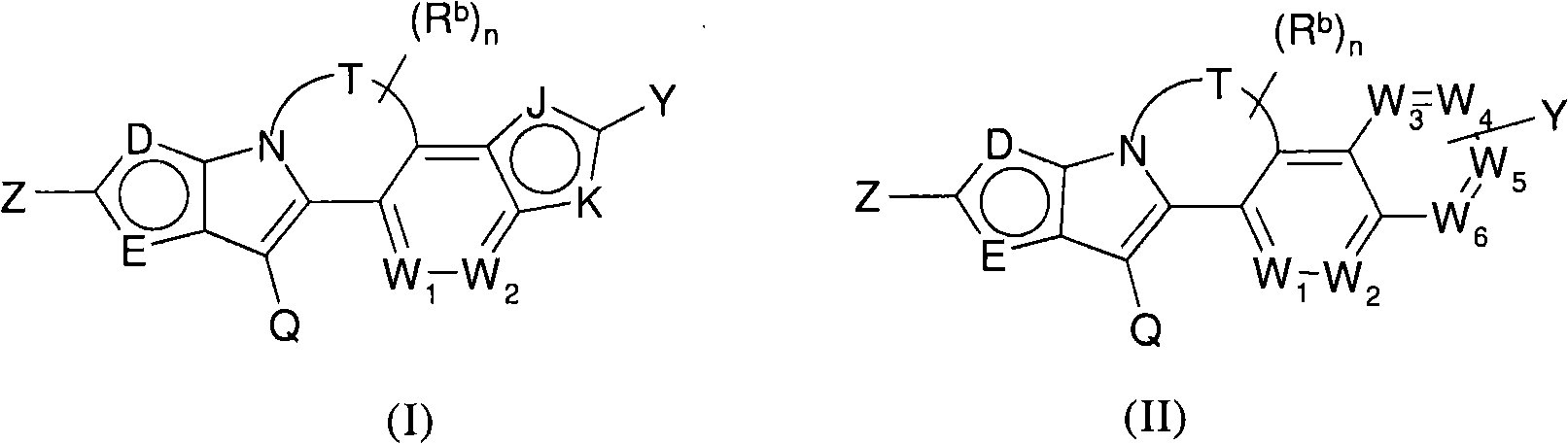
Polycyclic viral inhibitors
A technology of heterocyclyl and cycloalkyl, applied in the field of medicinal chemistry, can solve problems such as the lack of progress in compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
[0402] In the following examples and the above synthetic schemes, the following abbreviations have the following meanings. Where an abbreviation is not defined, it has its generally accepted meaning.
[0403] μL = microliter
[0404] μM = micromole
[0405] μg = microgram
[0406] NMR = nuclear magnetic resonance
[0407] br = broad peak
[0408] d = doublet
[0409] δ = chemical shift
[0410] dd = two sets of doublets
[0411] DMEM = Dulbeco's Modified Eagle's Medium (Dulbeco's
[0412] Modified Eagle's Medium)
[0413] DMF = N,N-Dimethylformamide
[0414] DMSO = dimethyl sulfoxide
[0415] DTT = dithiothreitol
[0416] EDTA = ethylenediaminetetraacetic acid
[0417] ESI = Electrospray Ionization
[0418] g = gram
[0419] h or hr = hour
[0420] HCV = hepatitis C virus
[0421] HPLC = High Performance Liquid Chromatography
[0422] Hz = hertz
[0423] IPTG = Isopropyl-β-D-thiogalactopyranoside
[0424] IU = International Unit
[0425] IC 50 ...
example 1
[0453] Synthesis of compound 225
[0454] Step 1: 3-Cyclohexyl-6-methylformate-1H-indole-2-boronic acid pinacol ester (10.2)
[0455]3g (8.9mmol) of 10.1, 6.8g (26.8mmol, 3eq) of bis(pinacolyl) diboron, 2.6g (26.5mmol, 3eq) of potassium acetate and 312mg (0.45mmol, 0.05eq) of Pd(PPh 3 ) 2 Cl 2 Pack into a 250mL reaction bottle. The mixture was then suspended with 60 mL of dioxane, and then degassed and flushed with argon (3 times). The reaction mixture was then heated gently to 90 °C for 16.5 h. HPLC analysis demonstrated complete consumption of 10.1. The reaction mixture was allowed to cool to room temperature and then concentrated. The crude residue was dissolved with EtOAc and passed through SiO 2 Chromatography (10→20% EtOAc in Hex) twice afforded 1.76 mg (52%) of 10.2 as a white powder. MS: 384.2 (M+H + ).
[0456] Step 2: Methyl 2-(1H-benzimidazol-2-yl)-3-cyclohexyl-1H-indole-6-carboxylate (10.3)
[0457] 100mg (0.26mmol) of 10.2, 64mg (0.33mmol, 1.25eq) of 2-...
example 2
[0461] Synthesis of compound 226
[0462] 97 mg (0.26 mmol) of 10.3 was charged to a reaction vessel and dissolved with 10 mL DMF. Then NaH (31 mg, 0.78 mmol, 3 eq, 60% in mineral oil) was added and the mixture was stirred at room temperature for 15 minutes. 1,4-Diiodobutane (43 μL, 0.33 mmol, 1.25 eq) was then added via syringe and stirring of the reaction mixture continued at room temperature. HPLC and LC-MS analysis demonstrated that 10.3 was completely consumed after 4 h, during which additional portions of NaH and 1,4-diiodobutane were added. The reaction mixture was poured into a 50 mL flask and concentrated. By adding cold H 2 O to induce precipitation to separate the methyl esters. The resulting solid was then collected by centrifugation and dissolved with 3 mL THF, 1 mL MeOH and 1 mL LiOH (1M, aq.). The reaction mixture was stirred at 50°C and monitored by HPLC analysis. Upon completion, the reaction mixture was neutralized with 0.5 mL HCl (2M, aq.) and concentr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com