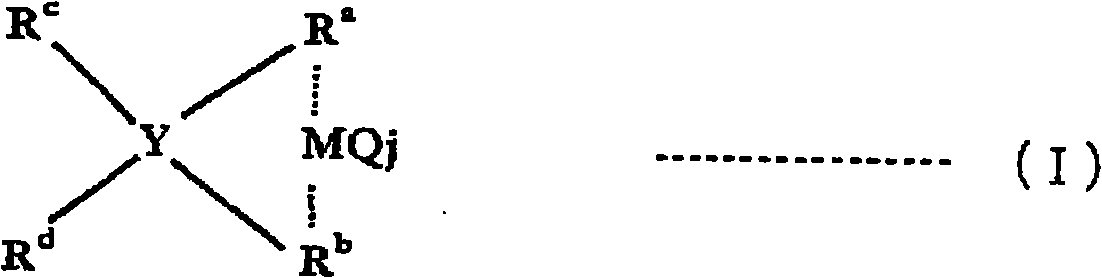Method for producing syndiotactic propylene polymer
A manufacturing method and technology of propylene, which is applied in the field of manufacturing syndiotactic propylene polymers, can solve the problems of undisclosed syndiotactic polypropylene, etc., and achieve the effect of high melting point
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
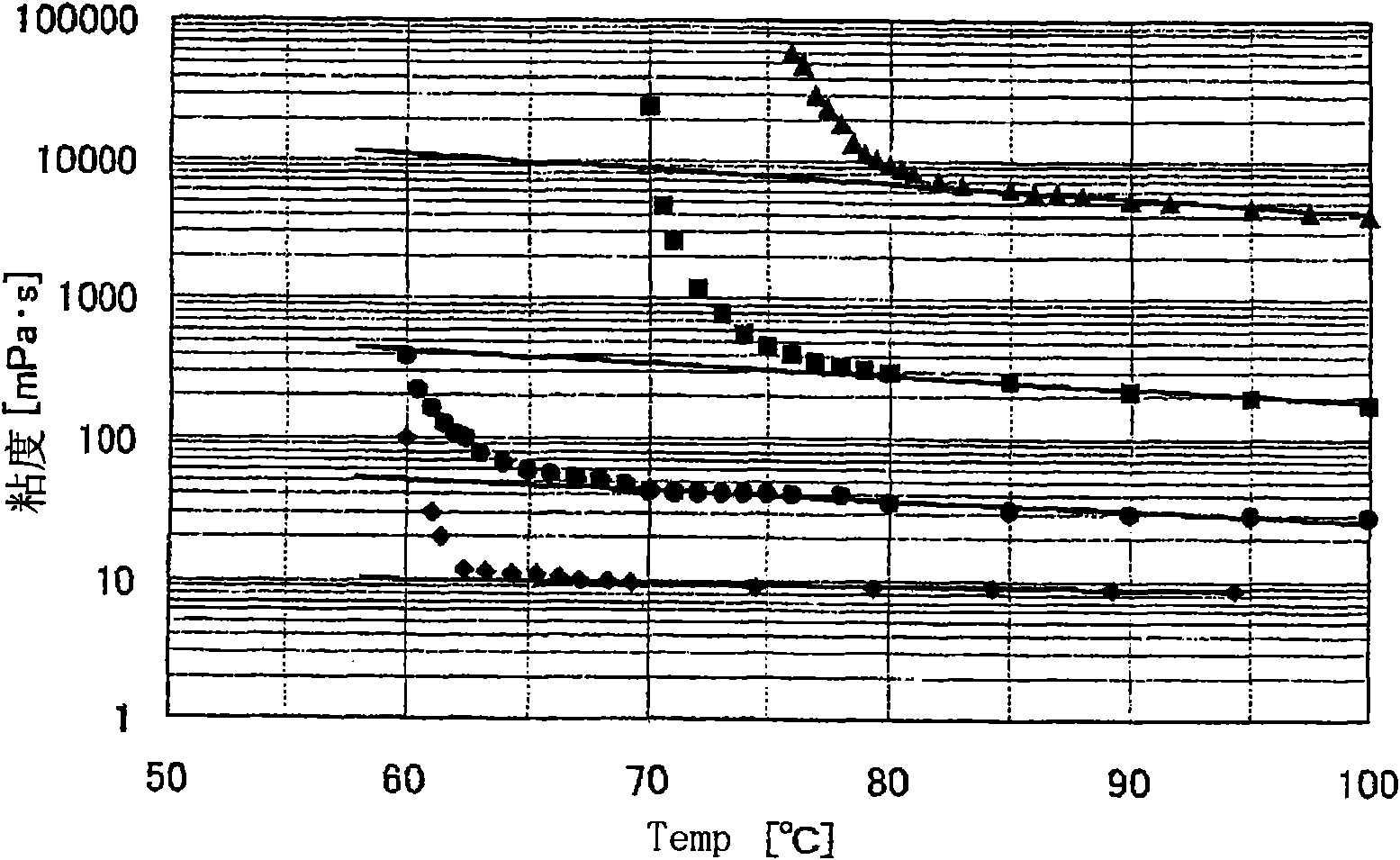
[0164] Hereinafter, the present invention will be described more specifically based on examples, but the present invention is not limited by these examples. However, various physical properties in Examples were measured as follows.
[0165] Melt flow rate (MFR)
[0166] According to the method of ASTM D-1238, it measured at 230 degreeC and the load of 2.16 kg.
[0167] Intrinsic viscosity [η]
[0168] It is the value measured at 135 degreeC in the decalin solvent. That is, about 20 mg of polymer is dissolved in 15 ml of decahydronaphthalene, and the specific viscosity η is measured in an oil bath at 135°C sp . In this decalin solution, add 5ml of decahydronaphthalene solvent to dilute, then measure the specific viscosity η by the same operation sp . Repeat this dilution operation and specific viscosity η again 2 times sp Determination of , extrapolating the concentration (C) to η at 0 sp The value of / C was obtained as an intrinsic viscosity (refer to the following...
Synthetic example 1
[0187] Dibenzylmethylene(cyclopentadienyl)(2,7-diphenyl-3,6-di-tert-butylfluorenyl)zirconium dichloride was produced as follows.
[0188] (i) Synthesis of 2,7-dibromo-3,6-di-tert-butylfluorene
[0189] Under a nitrogen atmosphere, in a 300 mL three-necked flask, 15.22 g (54.7 mmol) of 3,6-di-tert-butylfluorene synthesized according to the method described in Bull. 170 mL of propylene carbonate was stirred. To this solution, 20.52 g (115 mmol) of N-bromosuccinimide was added, followed by heating and stirring at 80° C. for 5 hours. Then, the reaction solution was left to cool naturally, and the reaction solution was added to 800 mL of water, and stirred at room temperature for 15 minutes, and the precipitated solid was separated by filtration. The resulting solid was washed 5 times with 10 mL of ethanol. Then, a mixed solution of n-hexane and a small amount of dichloromethane was added to the solid, heated to 60°C, and after the solid was completely dissolved, it was left s...
Embodiment 1
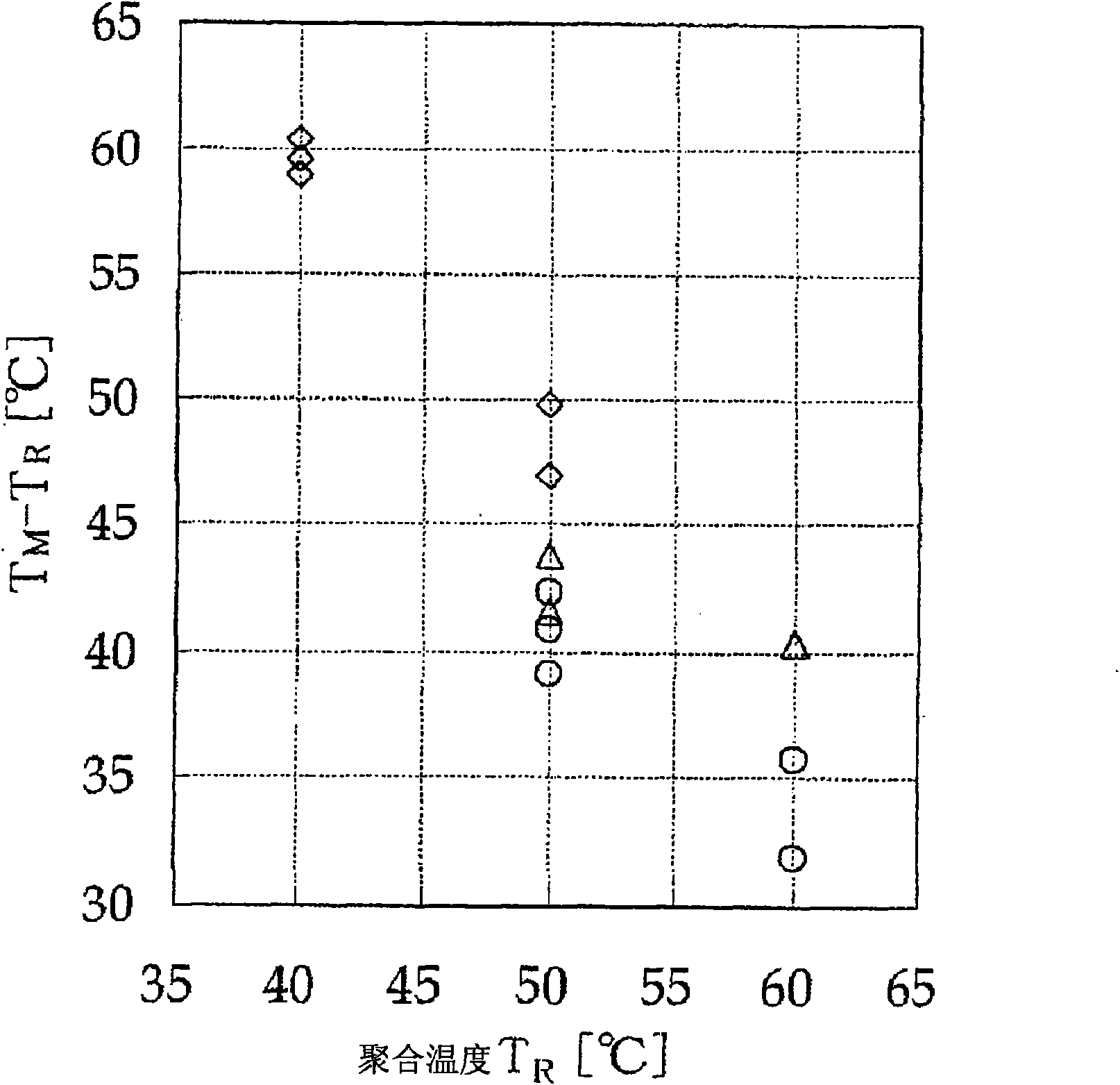
[0208] 150 mL of cyclohexane and 150 mL of n-heptane were placed in a 1 L stainless steel autoclave sufficiently replaced with nitrogen, and propylene was circulated at 30 L / hr, and kept at 45° C. for 20 minutes. On the other hand, a magnetic stirrer was placed in a 30 mL distillation flask fully replaced with nitrogen, and the dibenzylmethylene (cyclopentadienyl) (2,7-di Phenyl-3,6-di-tert-butylfluorenyl) zirconium dichloride 0.5μmol, TMAO-341 (manufactured by TOSOH FINECHEM company) hexane slurry (3.46mol / L in terms of aluminum atoms) 212.3mmol, then add n-heptane, stirred for 1 hour. This solution was added to a mixed solvent of cyclohexane and n-heptane (volume ratio = 1:1) in a stainless steel autoclave through which propylene was circulated, to initiate polymerization. Thereafter, only propylene was continuously supplied to maintain the total pressure at 0.5 MPa-G, and polymerization was performed at 50° C. for 1 hour. Since the exothermic control during polymerization...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melt flow index | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com