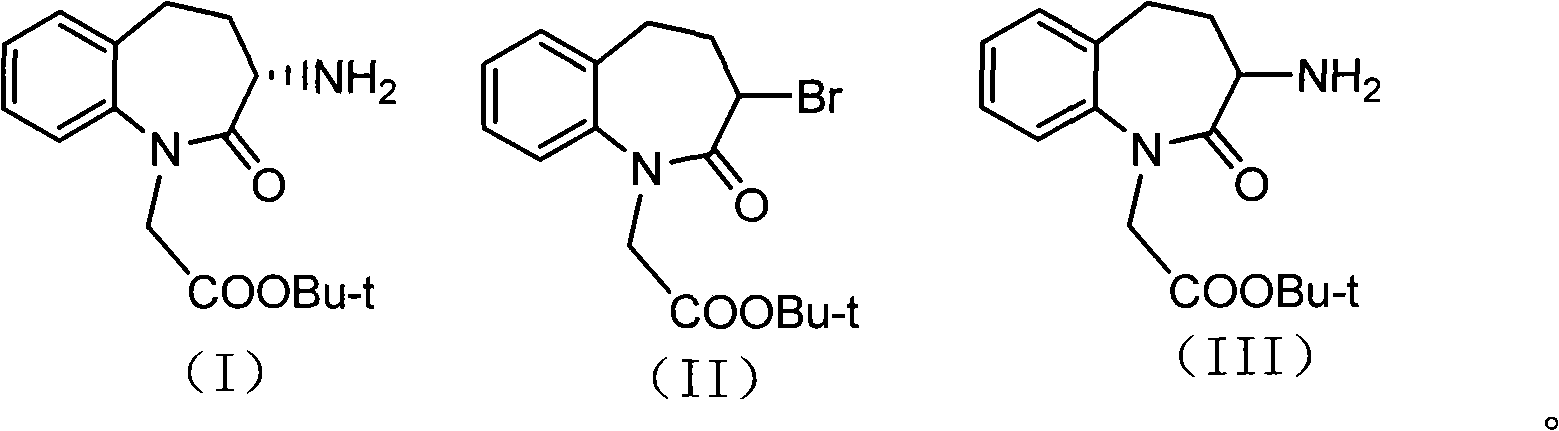
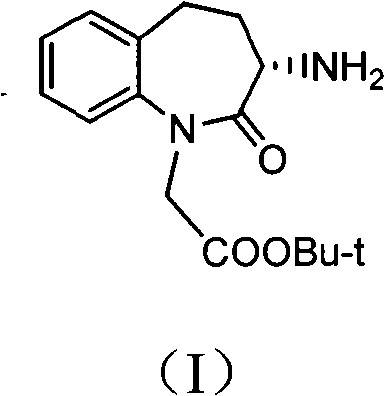
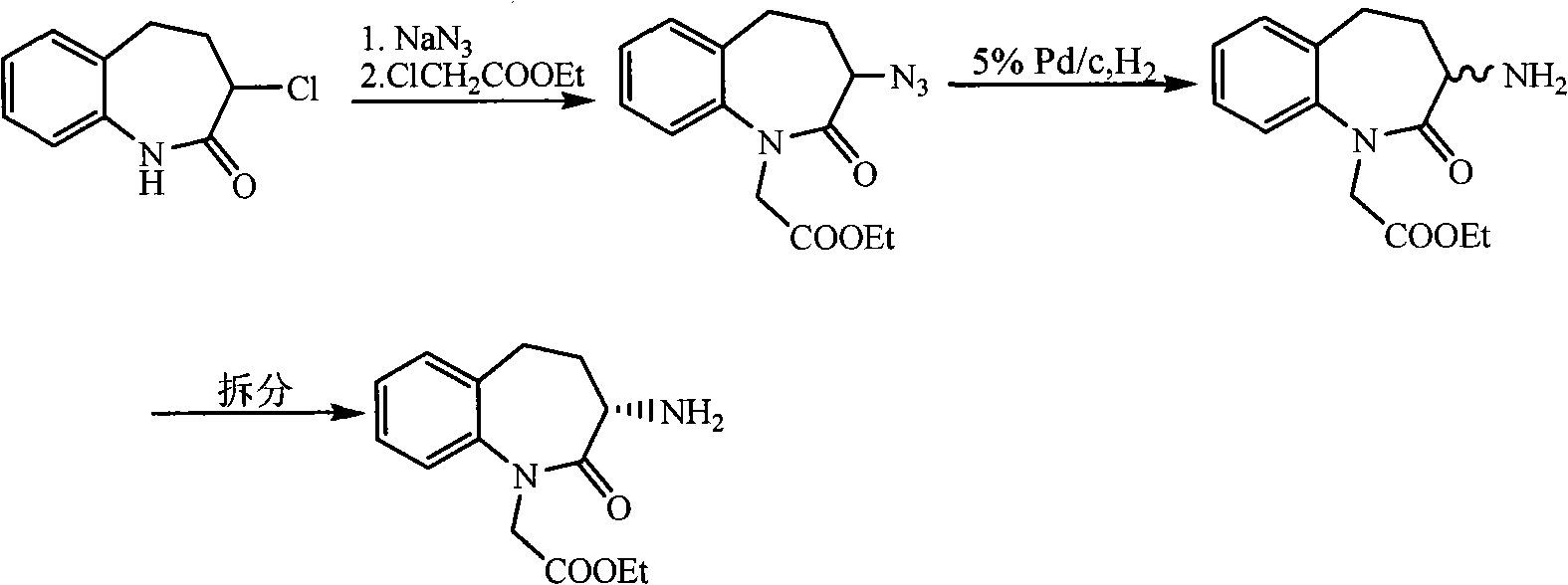
Synthetic method of Benazepril key intermediate S-amino substance
A synthesis method and intermediate technology are applied in the field of synthesis of key intermediates of Benazepril, which can solve the problems of poor atom economy and high cost, and achieve the effects of low pollution, high conversion rate and high optical purity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Take 250 grams of 3-bromo-2,3,4,5-tetrahydro-2-oxo-1H-1-benzazepine - 1-tert-butyl acetate, 2.5 grams of copper acetate and 3000ml of ethanol were added to the autoclave, and ammonia gas was introduced until the pressure was 5.0Mpa. After stirring at room temperature for 2 hours, the temperature was raised to 50°C for 2 hours. After the reaction finishes, filter cake and filtrate to obtain filter cake, filter cake reclaims copper acetate, after filtrate evaporates ethanol to dryness, residue is recrystallized with ethyl acetate, obtains 3-amino-2,3 of 176g (S, R) configuration, 4,5-Tetrahydro-2-oxo-1H-1-benzazepine - tert-Butyl 1-acetate. Yield: 85.9%, liquid phase purity 98.1%, optical rotation 0.
Embodiment 2
[0041] Take 75 grams of 3-bromo-2,3,4,5-tetrahydro-2-oxo-1H-1-benzazepine - 1-tert-butyl acetate, 3.75g of copper sulfate, and 750ml of methanol were added to the autoclave, and ammonia gas was introduced until the pressure was 3.0Mpa. After stirring at room temperature for 1 hour, the temperature was raised to 80°C for 1h. After the reaction, the filter cake and filtrate were obtained by filtration, and copper sulfate was recovered from the filter cake. After the filtrate was evaporated to dryness of ethanol, the residue was recrystallized with ethyl acetate to obtain 50.7 g of 3-amino-2,3 in the (S, R) configuration. , 4,5-Tetrahydro-2-oxo-1H-1-benzazepine - tert-Butyl 1-acetate. Yield: 82.5%, liquid phase purity 99.7%, optical rotation 0.
Embodiment 3
[0043] Take 50 grams of 3-bromo-2,3,4,5-tetrahydro-2-oxo-1H-1-benzazepine -1-Tert-butyl acetate, 5.0 grams of copper bromide, and 400 ml of isopropanol were added to the high-pressure reactor, and ammonia gas was introduced until the pressure was 2.5 Mpa. After stirring at room temperature for 3 hours, the temperature was raised to 120°C for reaction 3h. After the reaction was finished, the filter cake and filtrate were filtered, and the filter cake reclaimed copper bromide. After the filtrate was evaporated to dryness of ethanol, the residue was recrystallized with ethyl acetate to obtain 32.0g (S, R) configuration of 3-amino-2. 3,4,5-Tetrahydro-2-oxo-1H-1-benzazepine - tert-Butyl 1-acetate. Yield: 78.1%, liquid phase purity 97.9%, optical rotation 0.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com