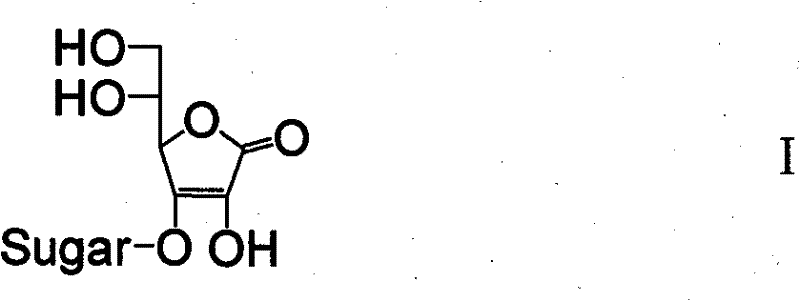
Ascorbic acid derivates, their preparation methods, intermediates and uses in cosmetics
A technology of ascorbic acid and derivatives, applied in cosmetics, cosmetics, sugar derivatives, etc., which can solve the problems of no superiority in physiological activity and no obvious improvement in stability, and achieve good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] Preparation of 1-Bromohepta-O-acetyllactose (3a)
[0067]Add 180mL of acetic anhydride to a three-necked flask equipped with a thermometer and a dropping funnel, cool in an ice-salt bath to 0°C, slowly add 0.6mL of perchloric acid dropwise, and control the internal temperature at 0-5°C, remove the ice after adding salt bath. At room temperature, 50.0 g of anhydrous lactose was added in batches, and the internal temperature was controlled at 33°C. After the addition, the reaction solution was cooled to 10°C, and 7.5g of red phosphorus was added. After stirring and dispersing, 14.5mL of bromine was added dropwise to the reaction solution. The internal temperature was lower than 20°C. Add 10.0mL of ice water to control the temperature of the reaction solution below 15°C. After dropping, stir at room temperature for 2.0h, pour into ice water, extract with chloroform several times, combine the organic phases, dry over anhydrous magnesium sulfate, and concentrate Dissolve t...
Embodiment 2
[0069] Preparation of 5,6-O-isopropylidene-L-ascorbic acid (7)
[0070] Add 91.0g of ascorbic acid and 450ml of acetone to a dry 1L three-necked flask, cool in an ice-salt bath to -5°C, slowly add 200.0g of concentrated sulfuric acid dropwise, keep the internal temperature at 0-5°C, drop it in about 2.5h, continue stirring for 5.0 min, remove the ice-water bath, naturally warm up to room temperature, continue to react for 45min, the reaction solution turns from colorless to light yellow, filter with suction, wash the filter cake several times with a small amount of acetone until the pH value is neutral, and vacuum-dry the filter cake (50°C ) for 1-2h, 89.5g of white powdery solid was obtained, its m.p.: 215-217°C, yield: 80.2%.
Embodiment 3
[0072] Preparation of 3-O-(hepta-O-acetyl-D-lactosyl)-(5,6-O-isopropylidene)-L-ascorbic acid (4a)
[0073] In a dry 1L round bottom flask, add 79.0g of 1-bromohepta-O-acetyllactose (3a), 28.1g of 5,6-O-isopropylidene-L-ascorbic acid (7), 500ml of acetone, and stir to disperse Then, add 28.0g potassium carbonate and 1.0g TEBAC, heat overnight at 50°C, filter with suction, recover the solvent to obtain a light yellow oil, which is then dissolved in 200mL ethyl acetate, washed several times with 20ml saturated saline, and anhydrous Dry over sodium sulfate, recover ethyl acetate, and vacuum-dry the residue with an oil pump for 1 h to obtain 57.0 g of light yellow foamy solid, melting point 52.5-54.0°C, yield: 60.2%.
[0074] 1 HNMR (CDCl 3 , 400M) δ: 1.21 (6H, -CH 3 ), 2.03-2.21 (21H, -CH 3 ), 3.98 (2H, -CH 2 -), 4.32 (2H, -CH 2 -), 4.37 (2H, -CH 2 -), 4.45(1H, -CH-), 4.47(1H, -CH-), 4.49(1H, -CH-), 4.50(1H, -CH-), 4.52(1H, -CH-), 4.54( 1H, -CH-), 4.61(1H, -CH-), 4.65(1H, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com