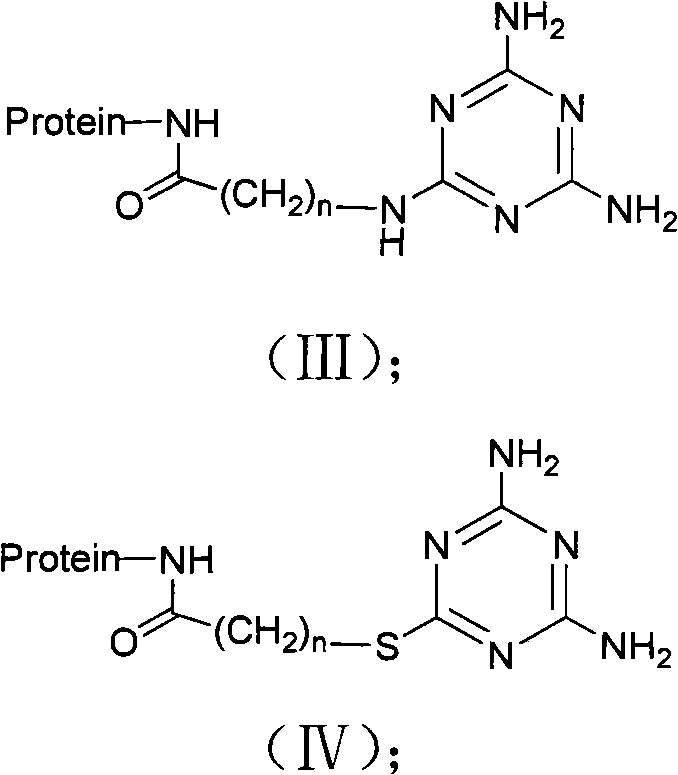
Method for preparing cyanide-urea triamide hapten, artificial antigen and antibody as well as application thereof
A technology of cyanuric triamide and artificial antigen, which is applied in chemical instruments and methods, receptors/cell surface antigens/cell surface determinants, testing dairy products, etc., to achieve the effect of safety monitoring
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
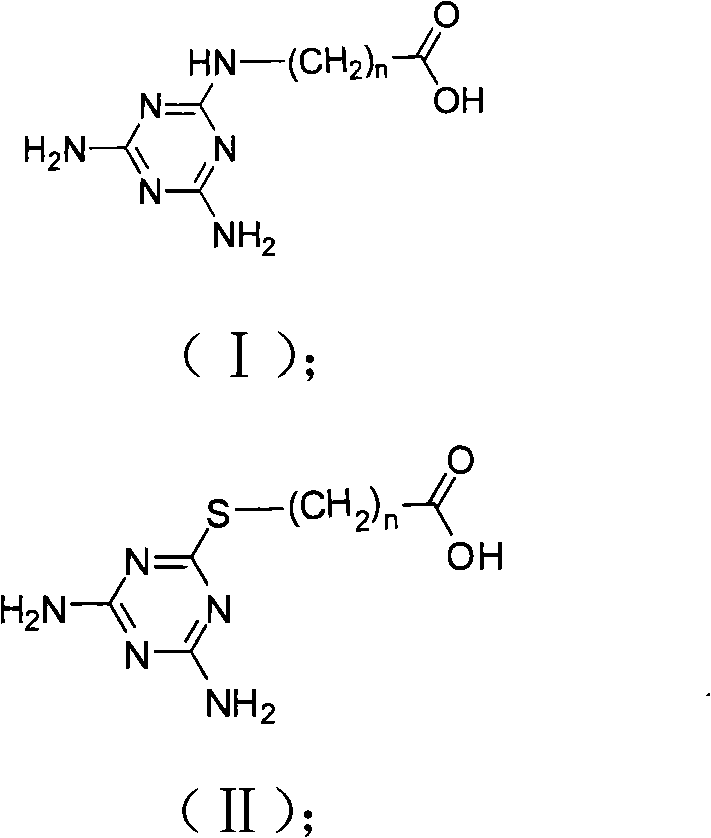
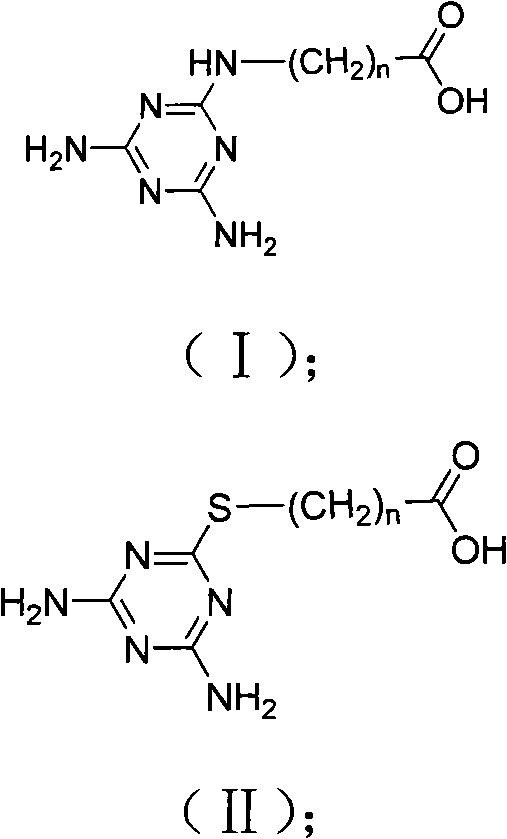
[0040] Example 1 Hapten 1 Synthesis
[0041] With 6-aminocaproic acid containing 6 carbon atoms as raw material, 5.4mmol of 2,4-diamino-6-chloro-1,3,5-triazine and 0.9mmol of 6-aminocaproic acid were packed into a circle In the bottom flask, add 100mL acetone, then add 2.7mmoL potassium hydroxide, react at 40°C for 48h, evaporate the solvent under reduced pressure to obtain a concentrate, dissolve the concentrate in 25mL of 10% aqueous sodium bicarbonate solution, and wash twice with n-hexane , and then the aqueous phase was acidified to pH 2 with concentrated acetic acid, and the precipitate was collected to obtain the hapten (CAAT-AC6).
[0042] Hapten identification results:
[0043] MS(EI), m / z(%): 240(M + , 11.68), 181 (50.88), 167 (17.28), 153 (39.01), 139 (100), 126 (31.09). to C 9 h 16 o 2 N 6 Carry out HRMS, the theoretical value is 2401329, and the measured value is 240.1329. IR(KBr)v max / cm -1 : 3492(s), 3409(s), 3331(s), 3159(s), 2937(s), 2860(w), 1642(v...
Embodiment 2
[0044] Example 2 Synthesis of Hapten 2
[0045] Take the aminopropionic acid containing 3 carbon atoms as a raw material example: put 5.4mmol of 2,4-diamino-6-chloro-1,3,5-triazine and 5.4mmol of aminopropionic acid into a round bottom flask , add 100mL ethanol, then add 10.8mmoL triethylamine, react at 70°C for 9h, evaporate the solvent under reduced pressure to obtain a concentrate, dissolve the concentrate in 25mL of 5% aqueous sodium bicarbonate solution, wash twice with dichloromethane, and then The aqueous phase was acidified with hydrochloric acid to a pH value of 1, and the precipitate was collected to obtain the hapten 3-(4,6-diamino-1,3,5-triazine-2-amino)-propionic acid (English name: 3-( 4,6-diamino-1,3,5-triazin-2-ylthio) propanoic acid), named M-a-3-BSA.
Embodiment 3
[0046] Example 3 Hapten 3 Synthesis
[0047] With 3-mercaptopropionic acid containing 3 carbon atoms as raw material, 5.4mmol of 2,4-diamino-6-chloro-1,3,5-triazine and 1.8mmol of 3-mercaptopropionic acid were loaded into the circle In the bottom flask, add 100mL tetrahydrofuran, then add 4.5mmoL potassium hydroxide, react at 60°C for 38h, evaporate the solvent under reduced pressure to obtain a concentrate, dissolve the concentrate in 25mL 6% aqueous sodium bicarbonate solution, and wash twice with petroleum ether , and then the aqueous phase was acidified with hydrochloric acid to a pH value of 2, and the precipitate was collected to obtain a hapten.
[0048] When amino acids or mercapto acids with carbon chains of other lengths are used as raw materials, the synthesis method is the same as in Examples 1-3.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com