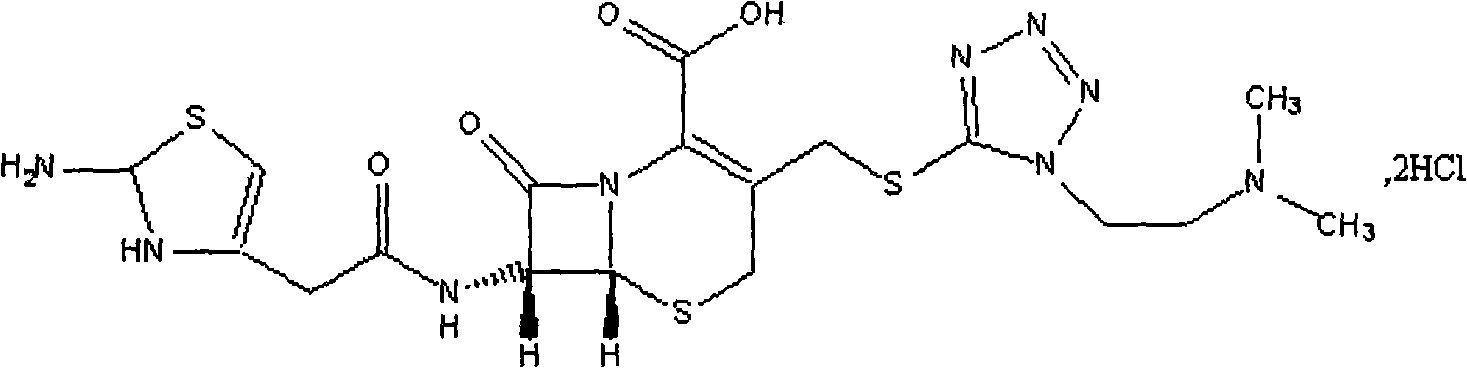
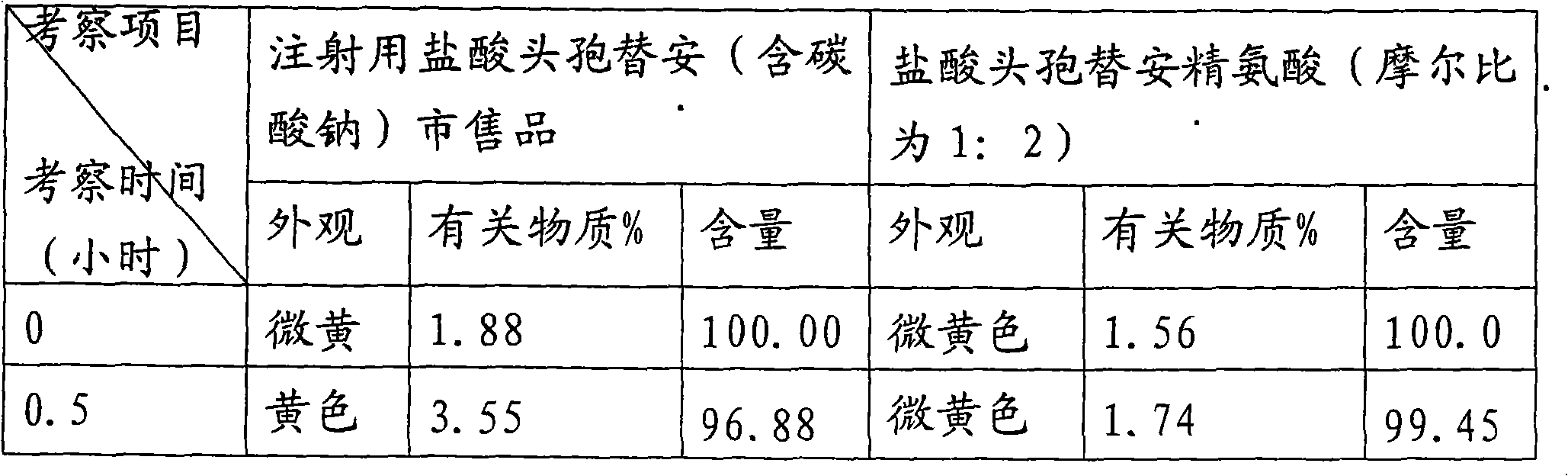
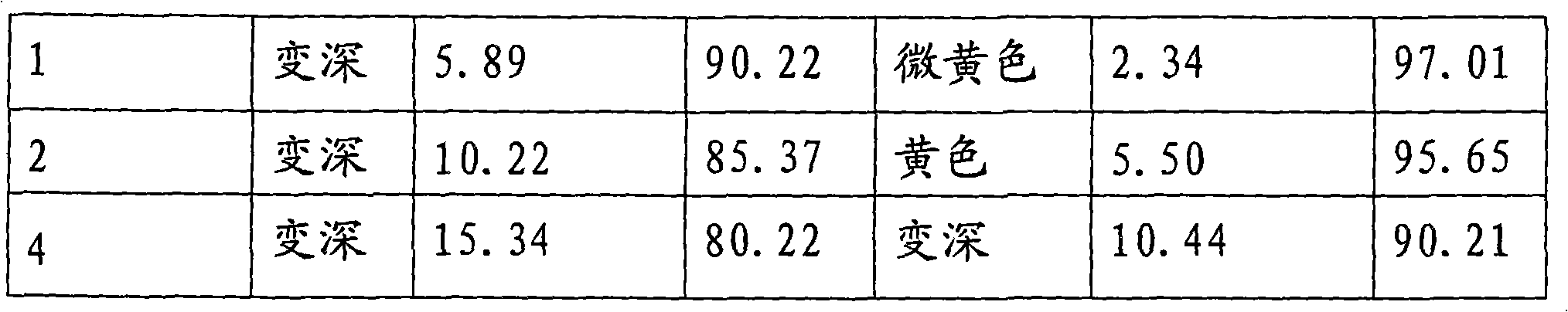
Medicament composition of cefotiam hydrochloride and preparation thereof
A technology for cefotiam hydrochloride and pharmaceutical preparations, applied in the field of pharmaceutical compositions, can solve problems such as unfavorable use, inability to store for a long time, etc., and achieve the effects of low side effects, stable product quality, and overcoming foam
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Cefotiam Hydrochloride 250g
[0034] Arginine 72.76g
[0035]
[0036] A total of 1000 bottles were made
[0037] Preparation Process:
[0038]Bottle washing: take the qualified vials, put them neatly in the stainless steel turntable, and transfer to the bottle washing station. Rinse with purified water and water for injection in turn, blow off residual moisture with compressed air, and enter a 350°C tunnel oven for drying, sterilization, and cooling. Go to the subpackaging station, and the mixture of cefotiam hydrochloride and arginine is subpackaged into 1000 bottles.
[0039] Plug washing: Take the qualified chlorinated butyl rubber plug, remove the inner and outer packaging, pour it into the cleaning tank, rinse it with purified water for 20 minutes, then rinse it with water for injection for 20 minutes, dry it in a drying oven, and sterilize it at 122 °C for a period of time 40 minutes.
[0040] Sterilization of alu...
Embodiment 2
[0046] Cefotiam Hydrochloride 250g
[0047] Arginine 1455g
[0048]
[0049] A total of 1000 bottles were made
[0050] Preparation process: the preparation method is the same as in Example 1.
Embodiment 3
[0052] Cefotiam Hydrochloride 250g
[0053] Arginine 727.6g
[0054]
[0055] A total of 1000 bottles were made
[0056] Preparation process: the preparation method is the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com