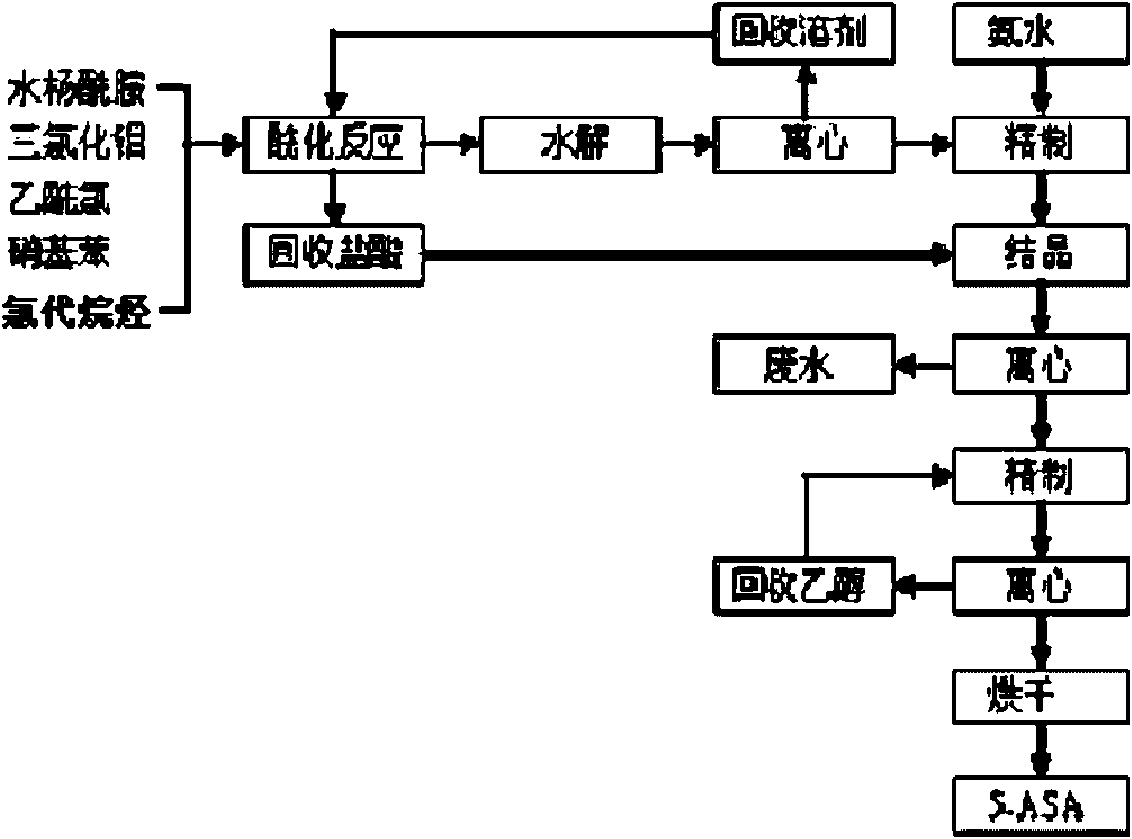
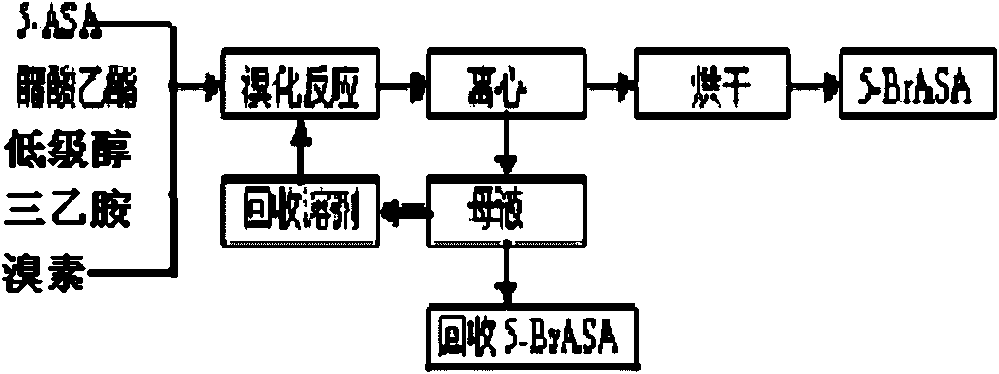
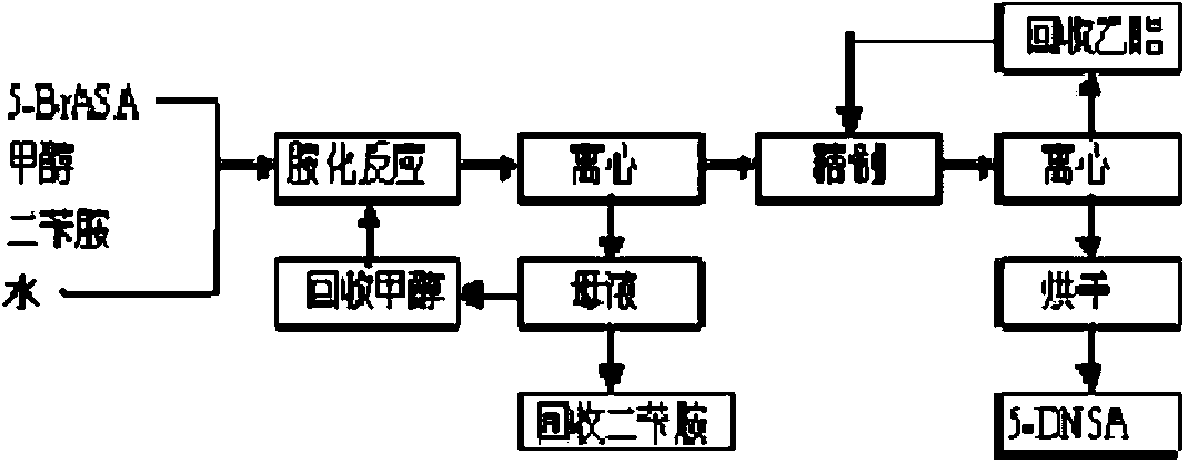
Method for preparing 5-(N,N-dibenzylglycyl) salicylamide
A technology of dibenzylaminoacetyl and acetylsalicylic amide, applied in the field of preparation of 5-salicylic amide, can solve problems such as inability to satisfy, and achieve the effects of low product cost, convenient and abundant sources, and low impurity content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0079] Under normal pressure, put 800 kg of methanol and 400 kg of water into the amination kettle, put in 258 kg of 5-BrASA product, cool and control the temperature below 40°C, add dropwise a mixed liquid of 405 kg of dibenzylamine and 400 kg of methanol, and reflux at 65°C After 2 hours, cool to room temperature and centrifuge to obtain crude 5-DNSA, and the mother liquor obtained by centrifugation can be distilled to recover methanol and dibenzylamine. Put the crude product into the refining kettle, then put in 1000 kilograms of ethyl acetate, and then heat up the steam at 75° C. and reflux for 5 hours, then cool to normal temperature, and vacuum-dry at 100° C. after centrifugation to obtain 305 kilograms of 5-DNSA product (yield 85%). Ethyl acetate was recovered by distillation. The 5-DNSA product obtained in this example has a white powder appearance. After testing, its m.p. acid 0.05%, methyl 5-(N,N-dibenzylaminoacetyl)salicylate 0.02%.
Embodiment 2
[0081] Under normal pressure, put 800 kg of methanol and 500 kg of water into the amination kettle, put in 258 kg of 5-BrASA product, cool and control the temperature below 30°C, add dropwise a mixed liquid of 440 kg of dibenzylamine and 400 kg of methanol, and reflux at 65°C After 2 hours, cool to room temperature and centrifuge to obtain crude 5-DNSA, and the mother liquor obtained by centrifugation can be distilled to recover methanol and dibenzylamine. Put the crude product into the refining kettle, then put in 1000 kg of ethyl acetate, and then heat up the steam at 75° C. and reflux for 3 hours, then cool to normal temperature, and vacuum dry at 100° C. after centrifugation to obtain 320 kg of 5-DNSA product (yield 90%). Ethyl acetate was recovered by distillation. The 5-DNSA product obtained in this example has a white powder appearance. After testing, its m.p. Acid content was 0.07%, methyl 5-(N,N-dibenzylaminoacetyl) salicylate was 0.02%.
Embodiment 3
[0083] Under normal pressure, put 800 kg of methanol and 450 kg of water into the amination kettle, put 258 kg of 5-BrASA product into it, cool and control the temperature below 30°C, add dropwise a mixed liquid of 420 kg of dibenzylamine and 400 kg of methanol, and reflux at 65°C After 3 hours, cool to room temperature and centrifuge to obtain crude 5-DNSA, and the centrifuged mother liquor can be distilled to recover methanol and dibenzylamine. Put the crude product into the refining kettle, then put in 1000 kilograms of ethyl acetate, and then heat up the steam at 75° C. and reflux for 5 hours, then cool to normal temperature, and vacuum-dry at 100° C. after centrifugation to obtain 314 kilograms (yield 88%) of 5-DNSA product. Ethyl acetate was recovered by distillation. The 5-DNSA product obtained in this example has a white powder appearance. After testing, its m.p. Acid content was 0.06%, and methyl 5-(N,N-dibenzylaminoacetyl)salicylate was 0.01%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com