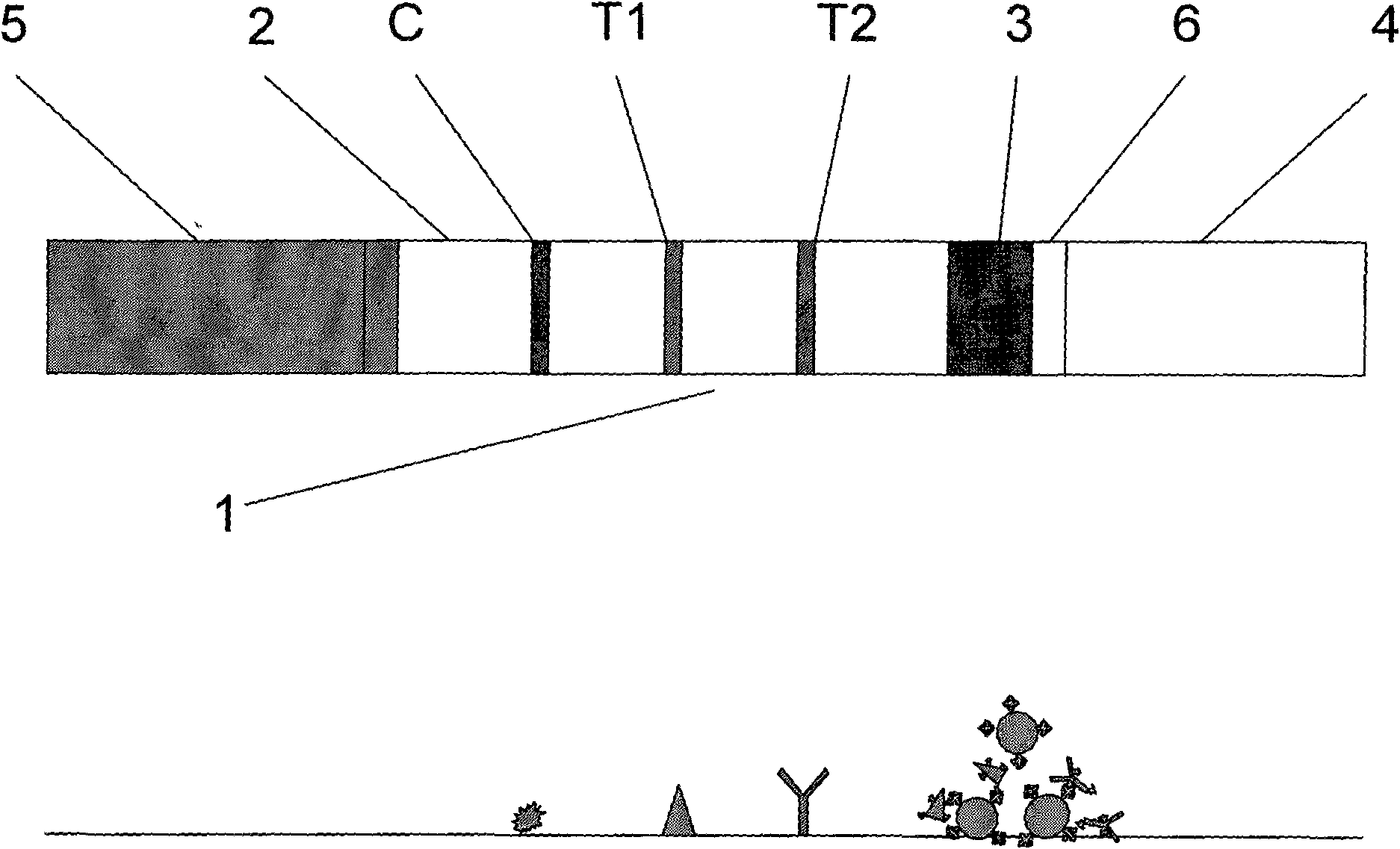
Magnetic immunochromatographic test strip for combined detection of HIV-1+2 antibody and P24 antigen and preparation method thereof
A magnetic immunochromatography and combined detection technology, applied in the field of medical testing, can solve the problems of long reaction time, long test period, limited application, etc., and achieve the effects of simplifying the preparation process, shortening the window period, and reducing subjectivity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Preparation method of magnetic immunochromatography test strip and test kit for combined detection of HIV-1+2 antibody and P24 antigen in blood
[0040] The preparation method of test strip and test paper box of the present embodiment comprises the following steps:
[0041] A. Preparation of antigen and antibody: select commercialized HIV1+2 recombinant antigen and P24 monoclonal antibody, dialyze against 20mM PBS with pH7.2 (pH7.0-7.6 is applicable), and dialyze overnight at 4°C for future use.
[0042] B. Preparation of coating film:
[0043] Coating buffer preparation: 0.02M phosphate buffer solution (PBS) with pH 7.2 was used as the coating buffer, which was sterilized by 0.22 μm microporous membrane and stored at 4°C for later use, with a validity period of two weeks.
[0044] Preparation of blocking solution: 0.02M phosphate buffered saline (PBS) containing 0.5% BSA at pH7.2 (pH7.0-7.6 is applicable), 0.22μm microporous membrane filter and sterilize, and store at...
Embodiment 2
[0072] Except; in the step of preparation of streptavidinized magnetic particles: streptavidin so that the ratio of streptavidin to magnetic particles is 1:2. Other steps are with embodiment 1,
Embodiment 3
[0074] In addition to the step of preparation of streptavidinized magnetic particles: streptavidin so that the ratio of streptavidin to magnetic particles is 1:10. Other steps are the same as in Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com