Negative light-sensitive resin combination
A technology of photosensitive resin and composition, applied in optics, opto-mechanical equipment, nonlinear optics, etc., can solve the problems of difficult to achieve high transmittance, colorization, volume shrinkage of interlayer insulating film, etc., and achieve UV transmission The effect of excellent rate, excellent chemical resistance, and excellent adhesion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
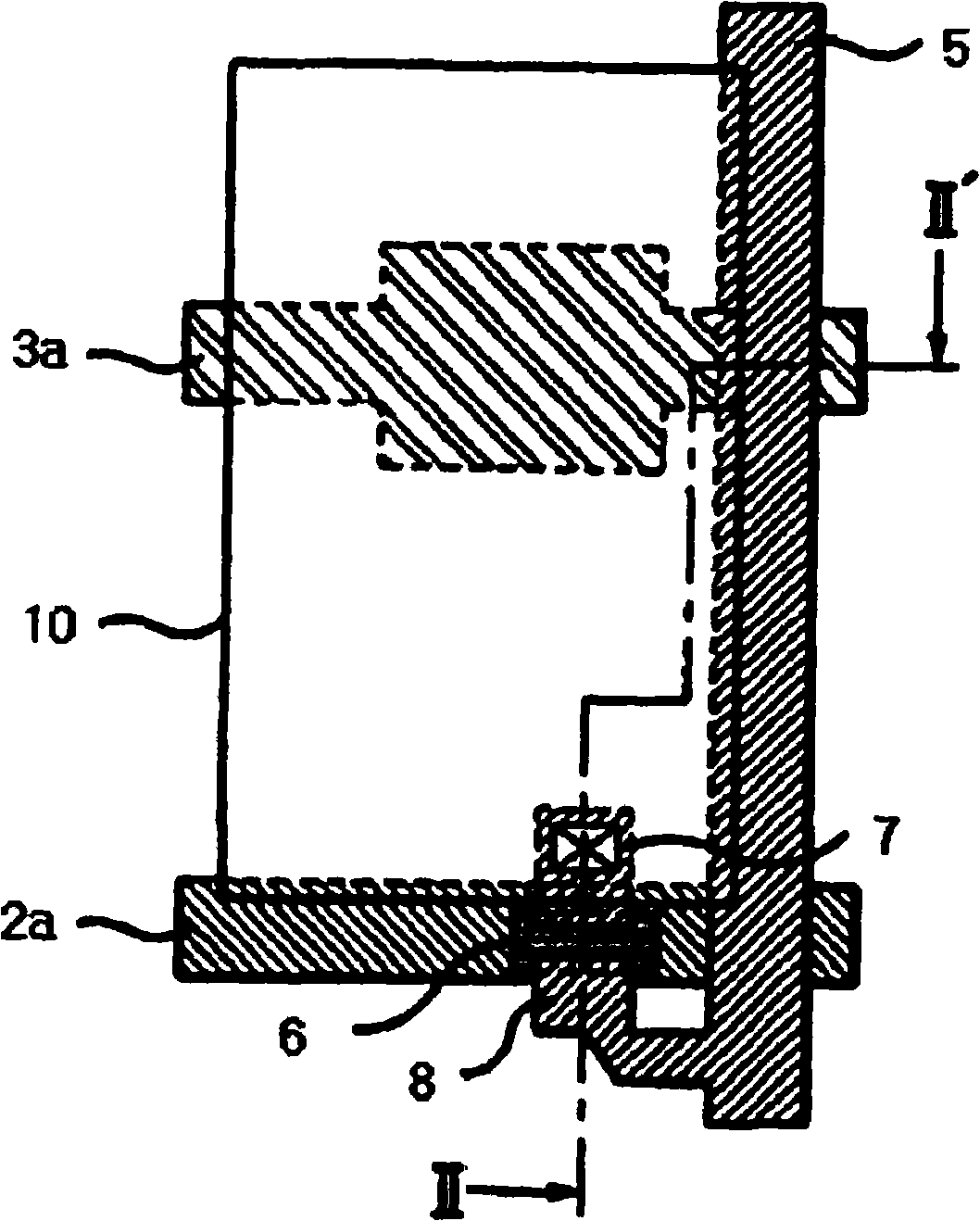
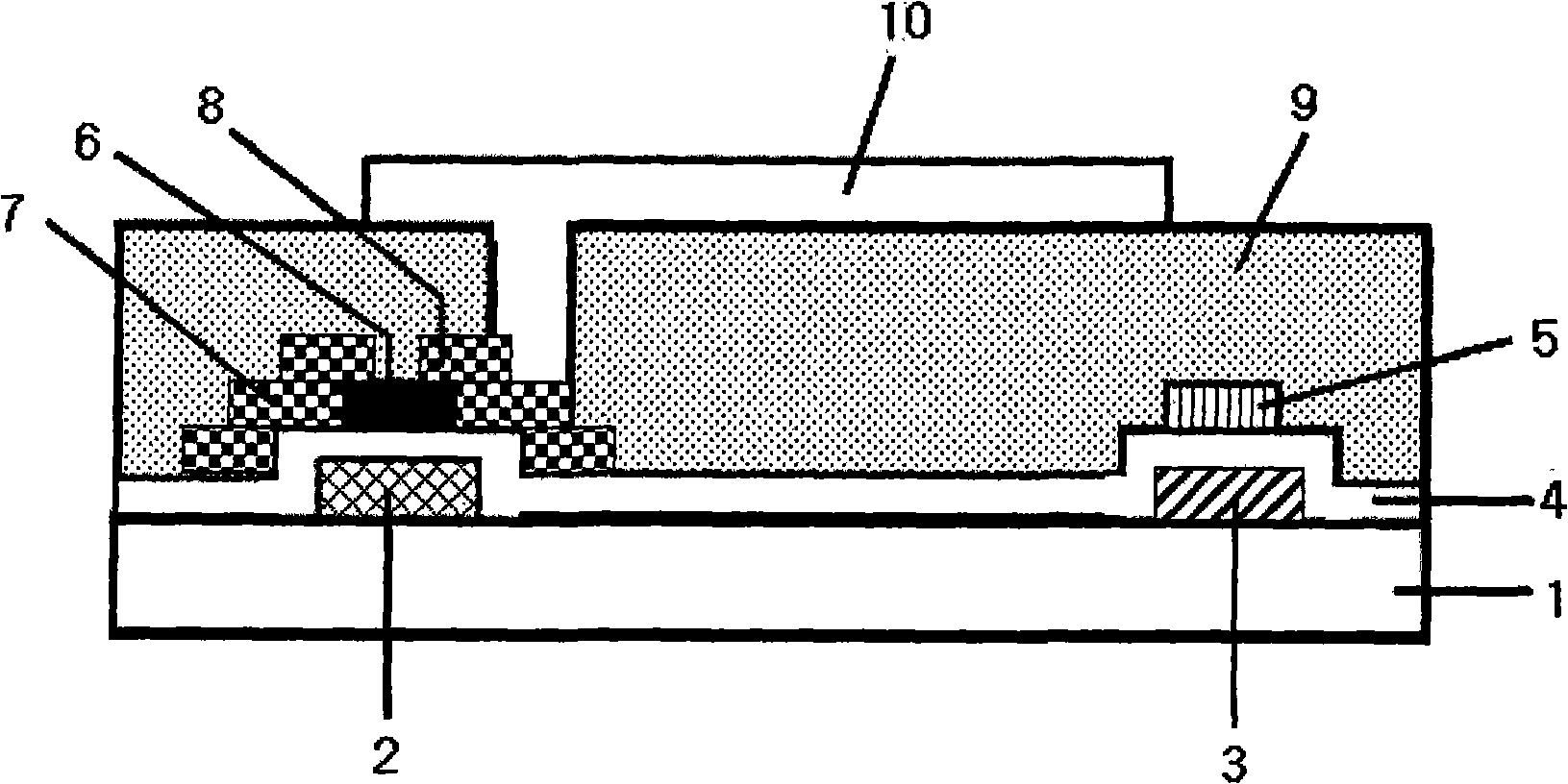
Image
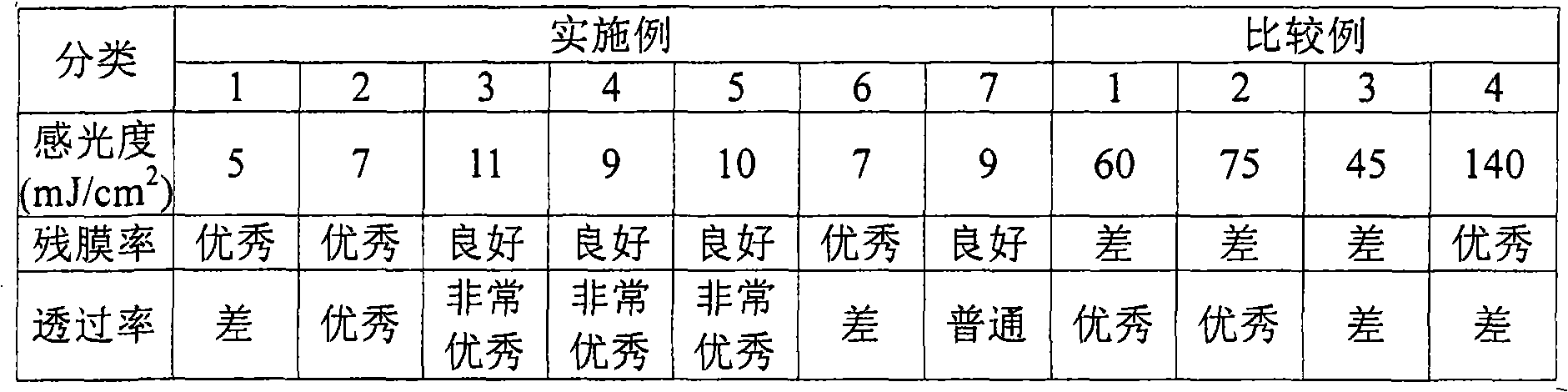
Examples
Embodiment 1
[0085] Example 1 (Manufacture of acrylic copolymer)
[0086] 400 parts by weight of isopropanol, 30 parts by weight of methacrylic acid, 30 parts by weight of styrene, 25 parts by weight of glycidyl methacrylate and 15 parts by weight of acrylic acid were placed in a 2L flask (Flask) equipped with a cooler and a stirrer - Mixed solution of 2-hydroxyethyl ester. The above liquid composition was thoroughly mixed at 600 rpm in a mixing vessel, and then 15 parts by weight of 2,2'-azobis(2,4-dimethylvaleronitrile) was added. The above-mentioned polymerization mixed solution was gradually raised to 50°C, and the temperature was maintained for 6 hours to obtain a copolymer solution. 500 ppm of phosphite as a polymerization inhibitor was added to the obtained polymer. The temperature of the flask in which the above-mentioned polymerization was stopped was lowered to 18° C. and left to stand for 1 hour, and the resulting precipitate was obtained and filtered. Propylene glycol mono...
Embodiment 2
[0090] Using 10 parts by weight of [1-[9-ethyl-6-(2-methylbenzoyl)-9H-carbazol-3-yl]-1-(O-acetyloxime) as a photoinitiator, in addition to Other than that, it implemented by the method similar to the said Example 1, and produced the negative photosensitive resin composition coating solution.
Embodiment 3
[0092] Using 5 parts by weight of [1-[9-ethyl-6-(2-methylbenzoyl)-9H-carbazol-3-yl]-1-(O-acetyloxime) as a photoinitiator, in addition to Other than that, it implemented by the method similar to the said Example 1, and produced the negative photosensitive resin composition coating solution.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com