Triazine carbonific containing aromatic chain structure and preparation method
A technology of triazine char-forming agent and chain structure, which is applied in the direction of fire-resistant coatings, etc., can solve the problems of large amount of organic solvent, complicated preparation process, and long reaction time, and achieve short reaction time, simple preparation process, and simple post-treatment method Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
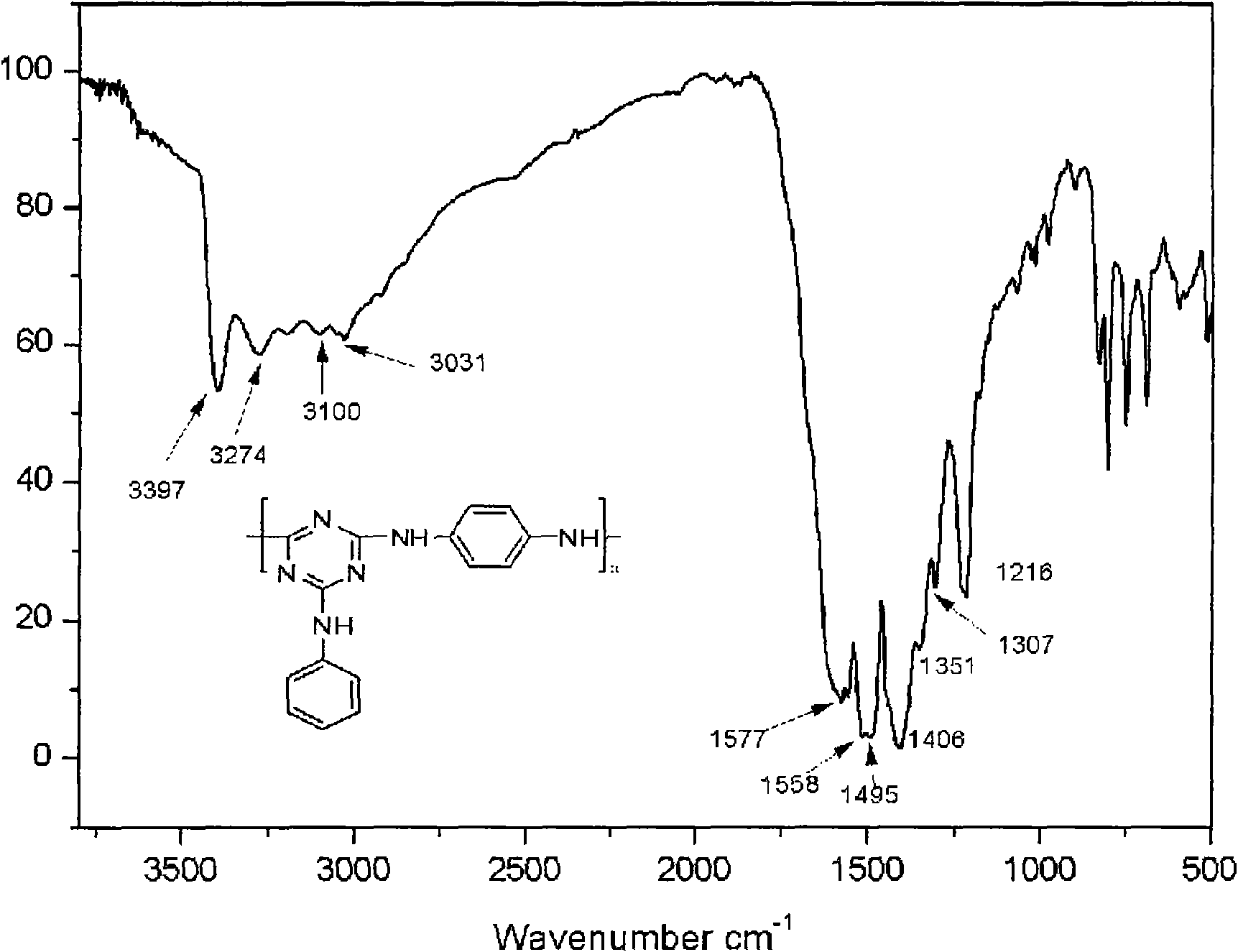
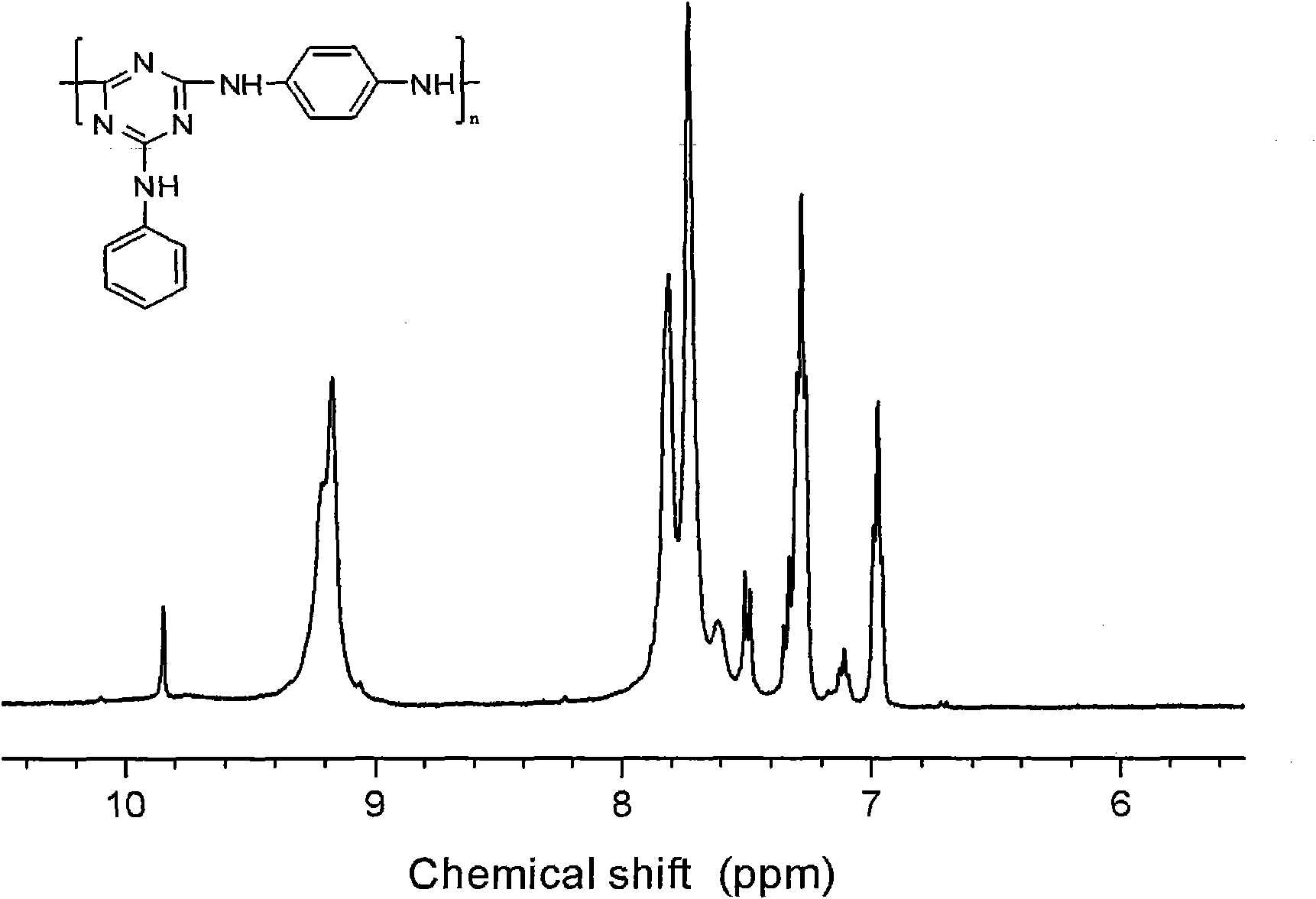
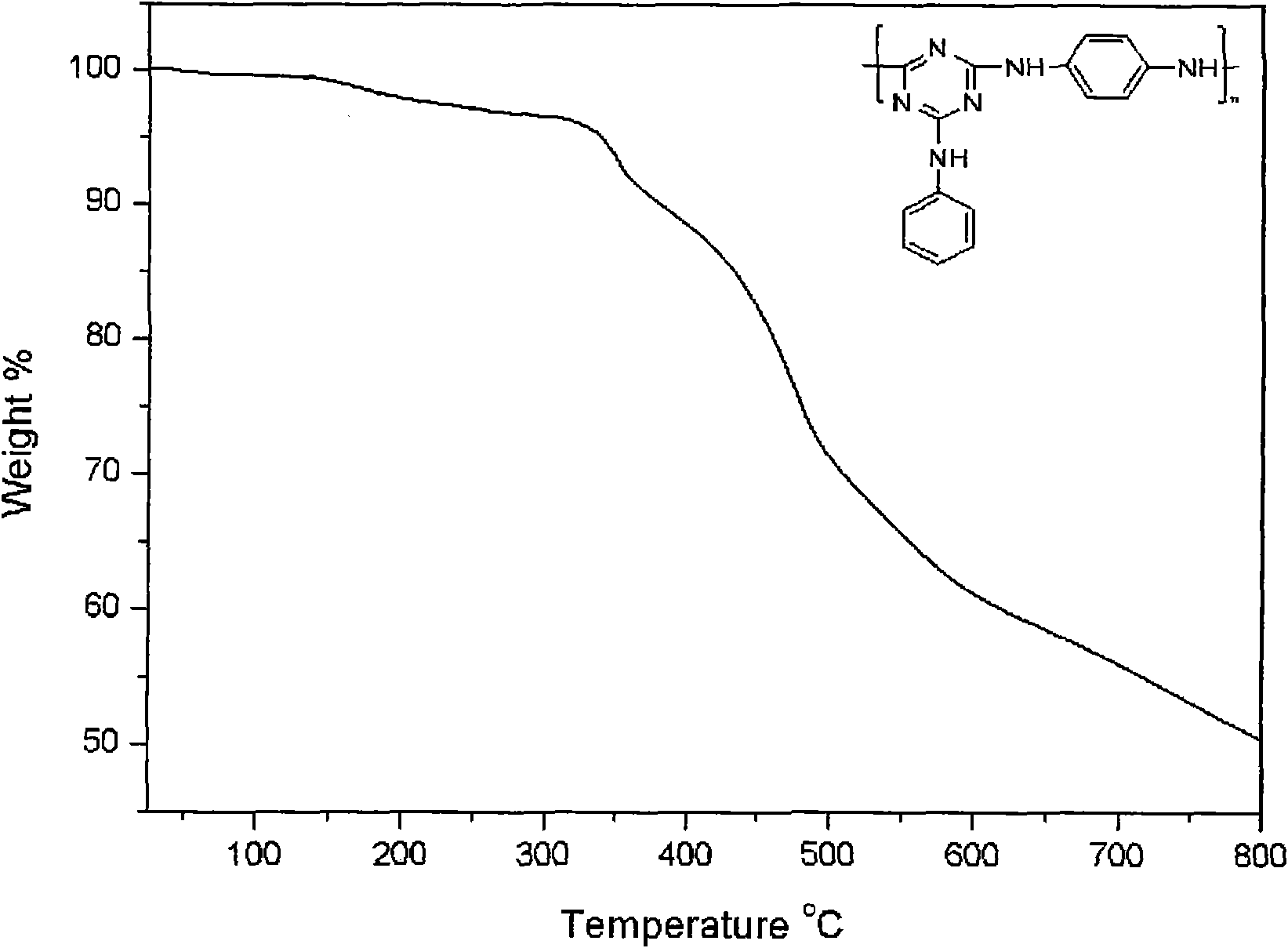
[0029] Embodiment 1: Under the protection of high-purity nitrogen, 1.84g cyanuric chloride is put into the reactor, adds 50ml N in the reaction vessel, N-dimethylacetamide, stirs 10-30 minute and makes cyanuric chloride The cyanide is evenly dispersed. Add 1.90ml N,N-diisopropylethylamine and 0.95ml aniline at 0-20℃. After reacting for 4 hours, add 1.90ml N,N-diisopropylethylamine to the reaction vessel successively. Ethylamine and 0.54 g p-phenylenediamine. Raise the reaction temperature to 45° C., stir and react for 4 hours, then add 1.90 ml of N,N-diisopropylethylamine and 0.54 g of p-phenylenediamine to the mixed solution in sequence. Raise the reaction temperature to 110°C, stir and react for 8-10 hours, then stop the reaction, cool, precipitate the product with water, filter with suction, wash with a large amount of water, dry in vacuum at 80°C for 48 hours, and obtain a three-dimensional aromatic chain structure Oxyzine macromolecule flame retardant. The yield is abov...
Embodiment 2
[0033] Embodiment 2: Under the protection of high-purity nitrogen, 1.84g cyanuric chloride is put into the reactor, and 50ml N, N-dimethylacetamide is added in the reaction vessel, and stirred for 10-30 minutes to make the cyanuric chloride The cyanide is evenly dispersed. Add 1.90ml N,N-diisopropylethylamine and 0.95ml aniline at 0-20℃. After reacting for 4 hours, add 1.90ml N,N-diisopropylethylamine to the reaction vessel successively. Ethylamine and 0.99 g of diaminodiphenylmethane. Raise the reaction temperature to 45° C., stir and react for 4 hours, then add 1.90 ml of N,N-diisopropylethylamine and 0.99 g of diaminodiphenylmethane to the mixed solution in sequence. Raise the reaction temperature to 110°C, stir and react for 8-10 hours, then stop the reaction, cool, precipitate the product with water, filter with suction, wash with a large amount of water, dry in vacuum at 80°C for 48 hours, and obtain a three-dimensional aromatic chain structure The oxazine macromolecule...
Embodiment 3
[0034] Embodiment 3: under the protection of high-purity nitrogen, put 1.84g cyanuric chloride into the reactor, add 50ml N in the reaction vessel, N-dimethylacetamide, stir 10-30 minute to make cyanuric chloride The cyanide is evenly dispersed. Add 1.38g of anhydrous potassium carbonate and 1.01g of diaminodiphenyl ether at 0-10°C. After reacting for 4 hours, add 1.38g of anhydrous potassium carbonate and 1.00g of diaminodiphenyl ether to the reaction vessel in turn. phenyl ether. Raise the reaction temperature to 55° C., stir and react for 4 hours, then add 1.38 g of anhydrous potassium carbonate and 1.00 g of diaminodiphenyl ether to the mixed solution in sequence. Raise the reaction temperature to 110°C, stir and react for 8-10 hours, then stop the reaction, cool, precipitate the product with water, filter with suction, wash with a large amount of water, dry in vacuum at 80°C for 48 hours, and obtain a three-dimensional aromatic chain structure The oxazine macromolecule f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com