Long-chain triazine macromolecular flame retardant with bicyclic phosphate structure and preparation method thereof
A bicyclic phosphate and triazine-based technology, applied in the direction of fire-resistant coatings, can solve the problems of mechanical and mechanical properties of materials, uneven distribution of components, moisture absorption of flame retardants, etc., to improve the mechanical behavior of materials, The preparation process is simple and the effect of high carbon formation rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
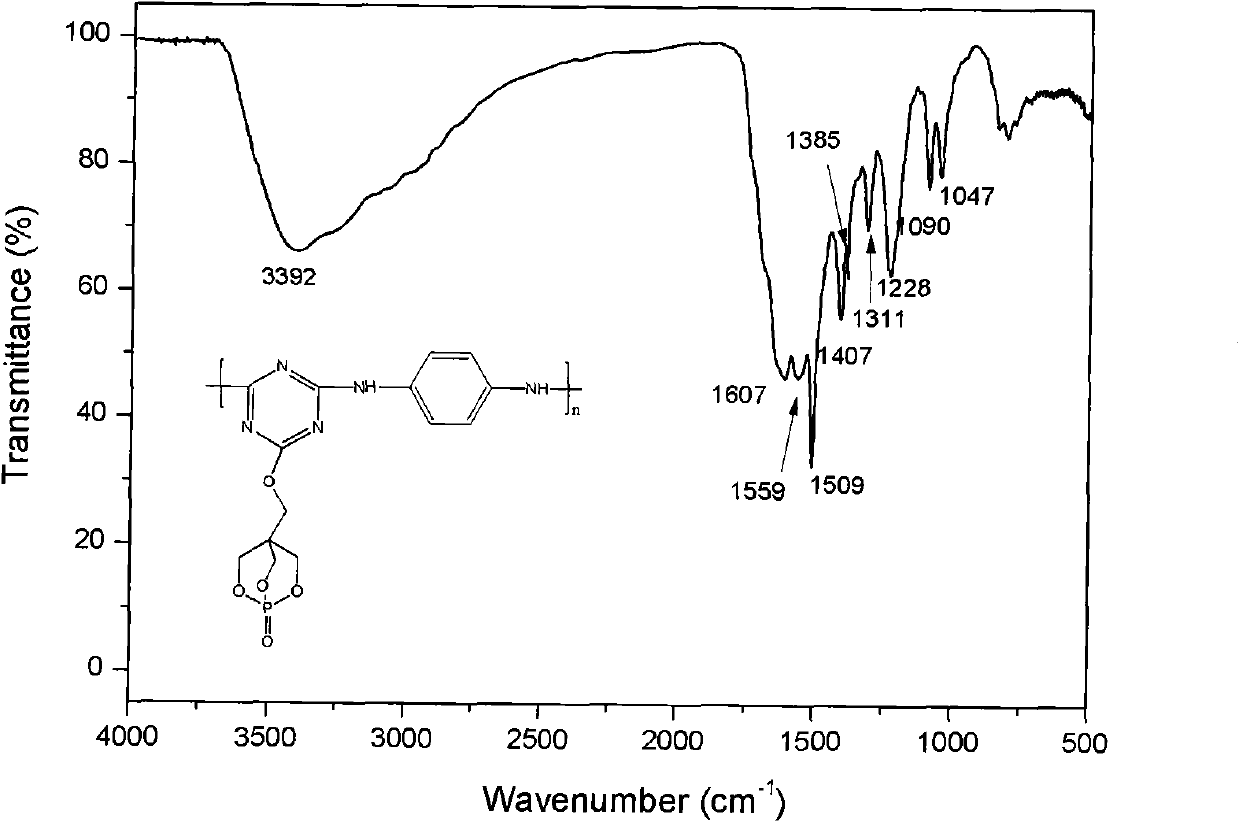
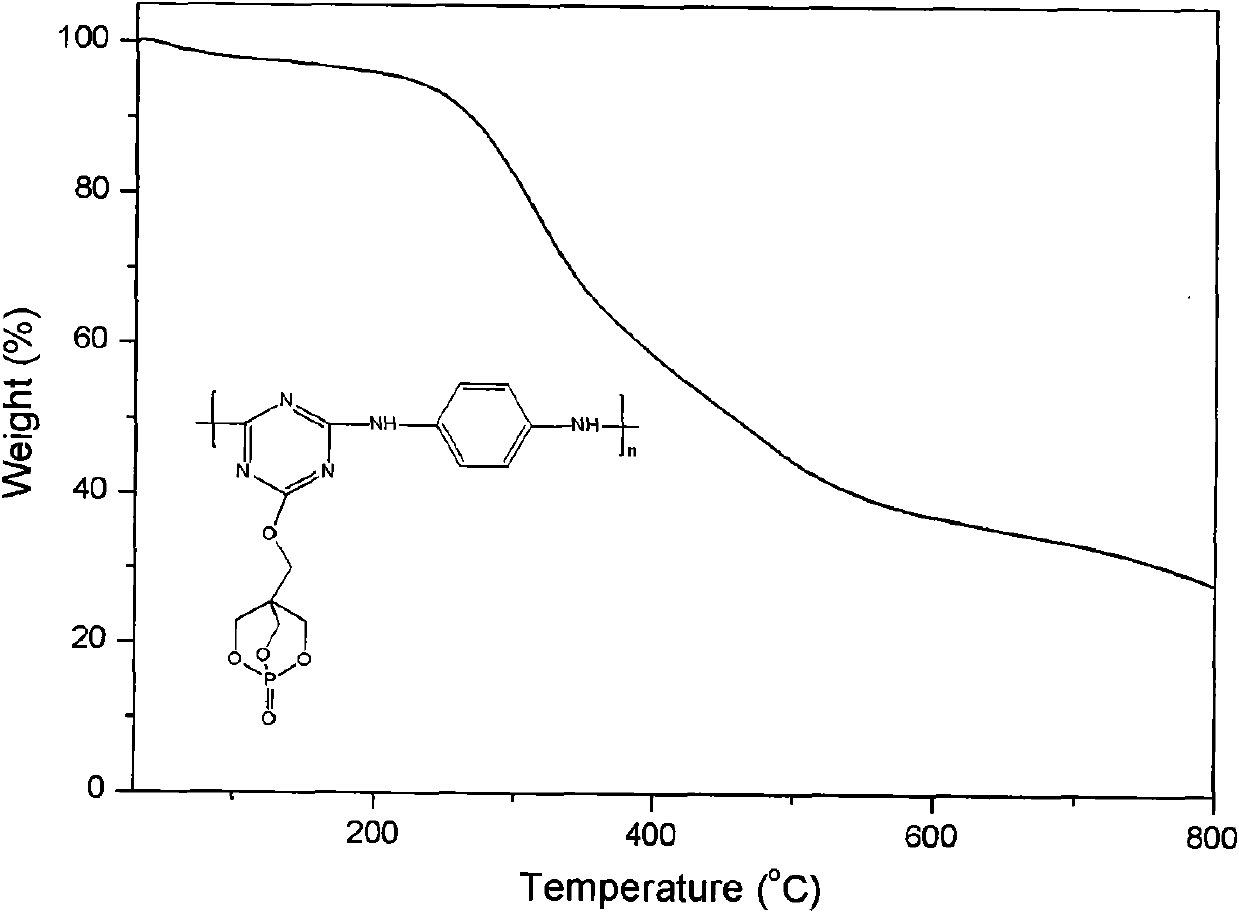
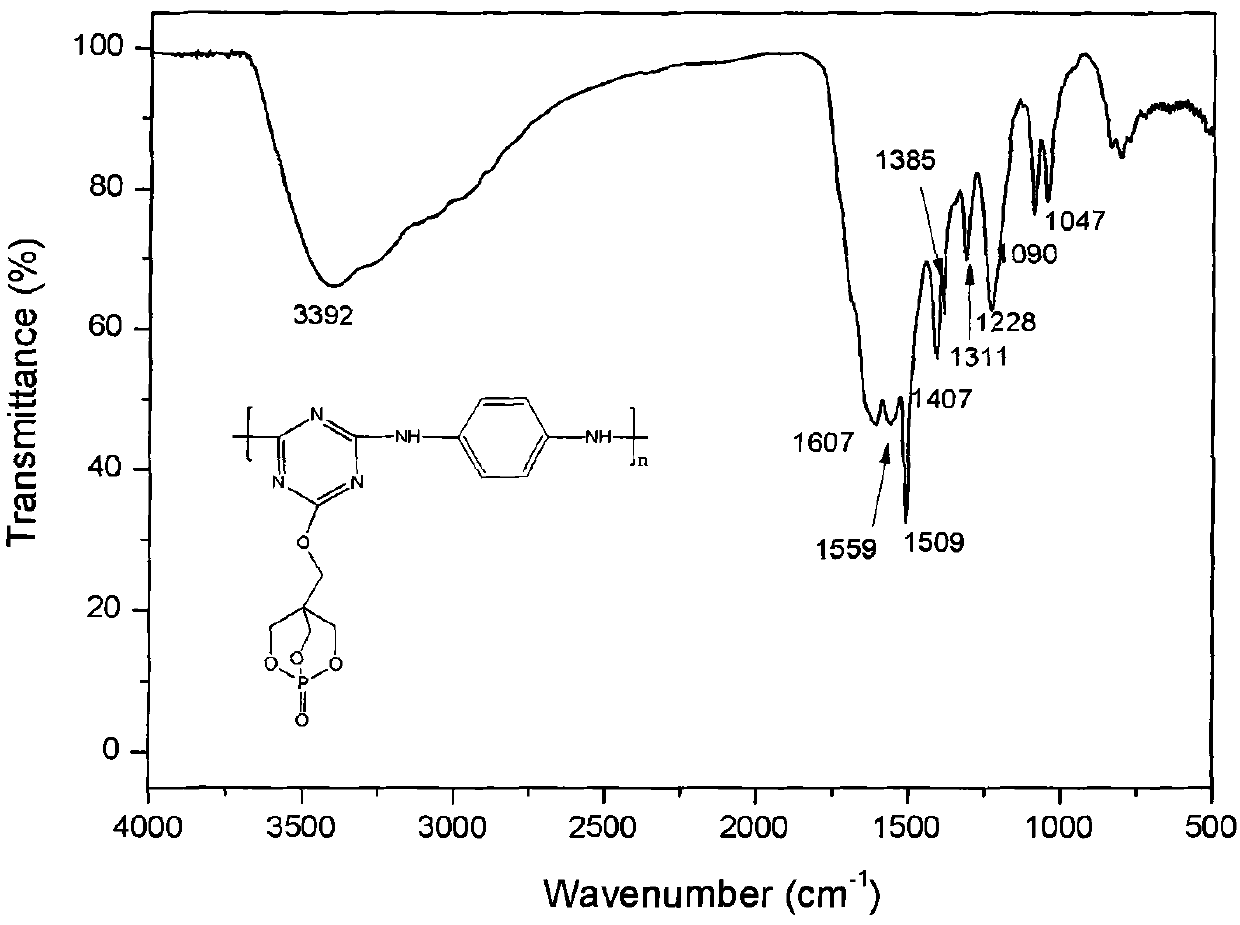
[0036] Embodiment 1: Under the protection of high-purity nitrogen, put 1.84g (10mmol) cyanuric chloride in the reactor, add 50mL N,N-dimethylacetamide in the reaction vessel, stir for 10-30 minutes to make The cyanuric chloride is evenly dispersed, after adding 2.09mL (12mmol) N, N-diisopropylethylamine and 1.80g (10mmol) PEPA, continue stirring for a period of time to make the reactants evenly disperse, react at 50°C for 24 hours, then 4.35 mL (25 mmol) of N,N-diisopropylethylamine and 1.08 g (10 mmol) of p-phenylenediamine were sequentially added to the reaction vessel. Raise the reaction temperature to 80°C, stir and react for 8 hours, then raise the reaction temperature to 120°C, stir and react for 10 hours, stop the reaction, cool, precipitate the product with acetone, filter with acetone, wash with a large amount of acetone, 80°C Dry under vacuum for 32 hours to obtain a long-chain triazine macromolecular flame retardant with a bicyclic phosphate structure.
Embodiment 2
[0037] Embodiment 2: under the protection of high-purity nitrogen, put 1.84g (10mmol) cyanuric chloride in the reactor, add 50mLN in the reaction vessel, N-dimethylacetamide, stir for 10-30 minutes to make three Polycyanogen chloride is dispersed evenly, after adding 2.09mL (12mmol) N, N-diisopropylethylamine and 1.80g (10mmol) PEPA, continue to stir for a period of time to make the reactants evenly dispersed, react at 50°C for 24 hours, and then add 4.35 mL (25 mmol) of N,N-diisopropylethylamine and 1.98 g (10 mmol) of diaminodiphenylmethane were sequentially added to the reaction vessel. Raise the reaction temperature to 80°C, stir and react for 10 hours, then raise the reaction temperature to 110°C, stir and react for 10 hours, stop the reaction, cool, precipitate the product with ethanol, filter with suction, wash with a large amount of ethanol, and put it under 80°C Vacuum drying for 48 hours to obtain a long-chain triazine macromolecular flame retardant with a bicyclic p...
Embodiment 3
[0038] Embodiment 3: under the protection of high-purity nitrogen, put 1.84g (10mmol) cyanuric chloride into the reactor, add 50mLN in the reaction vessel, N-dimethylacetamide, stir for 10-30 minutes to make three Polycyanogen chloride is dispersed evenly. After adding 1.66g of anhydrous potassium carbonate (12mmol) and 1.80g (10mmol) of PEPA, continue to stir for a period of time to make the reactants evenly dispersed. After 24 hours of reaction at 50°C, add 3.45g (25mmol) of anhydrous potassium carbonate and 2.00g (10mmol) of diaminodiphenyl ether. Raise the reaction temperature to 70°C, and stir the reaction for 6 hours. Raise the reaction temperature to 120°C, stir and react for 12 hours, stop the reaction, cool, precipitate the product with water, filter with suction, wash with a large amount of acetone, and dry in vacuum at 80°C for 48 hours to obtain a long-chain bicyclic phosphate ester structure Triazine macromolecular flame retardant.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com