Method for preparing dendritic macromolecule of polyester
A dendritic and macromolecular technology, applied in the field of polymer material preparation, can solve the problems of loss of dendritic macromolecules, cumbersome purification steps, low yield, etc., and achieve faster synthesis speed, better biocompatibility, improved quality and The effect of using security
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
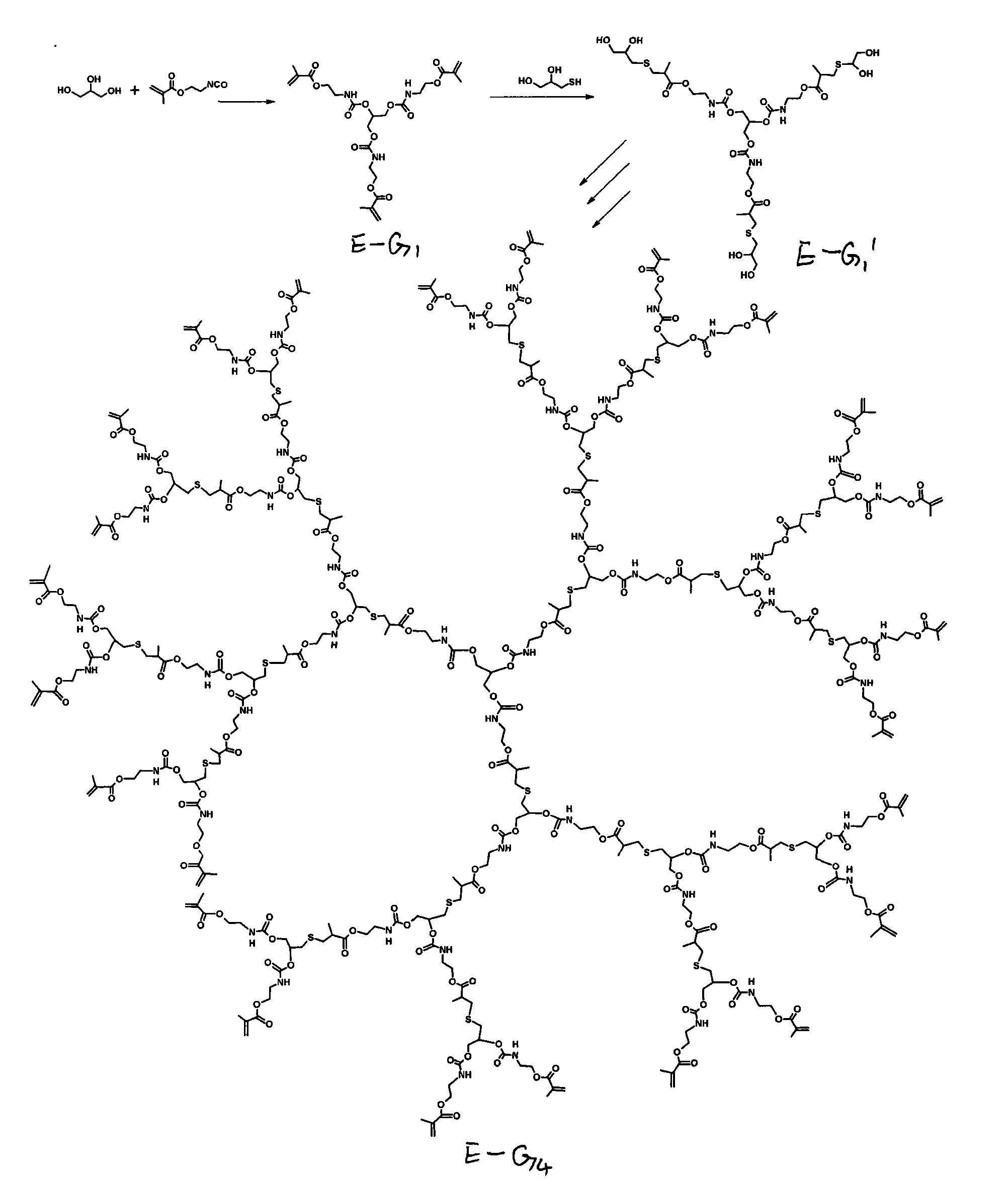
Image
Examples
Embodiment 1
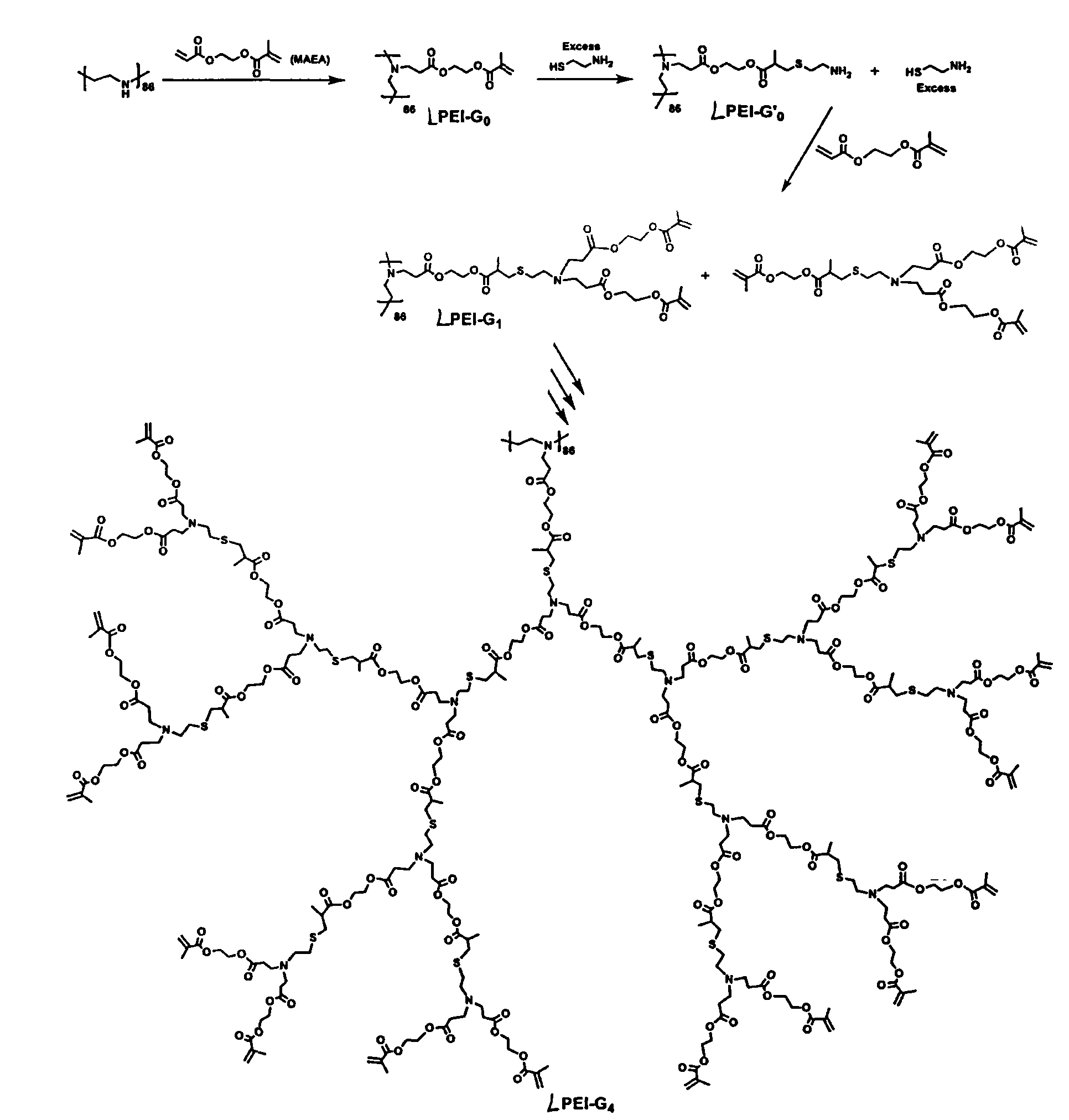
[0027] Example 1 Preparation of polyester dendrimers using chemical reaction kinetics asymmetry monomer pair MAEA and cysteamine
[0028] (1) Synthesis of monomer methacryloxy ethyl acrylate (MAEA)
[0029] Dissolve 78g (0.60mol) of hydroxyethyl methacrylate and 100ml (0.72mol) of triethylamine in 400ml of CH at 0°C 2 Cl 2 52.4ml (0.66mol) of acryloyl chloride was added dropwise, stirred overnight, and the precipitate was filtered off. The filtrate was washed 3 times with 150ml of water each time, and the organic phase was dried with sodium sulfate and spin-dried to obtain 104.2g of MAEA with a yield of 94.3 %.
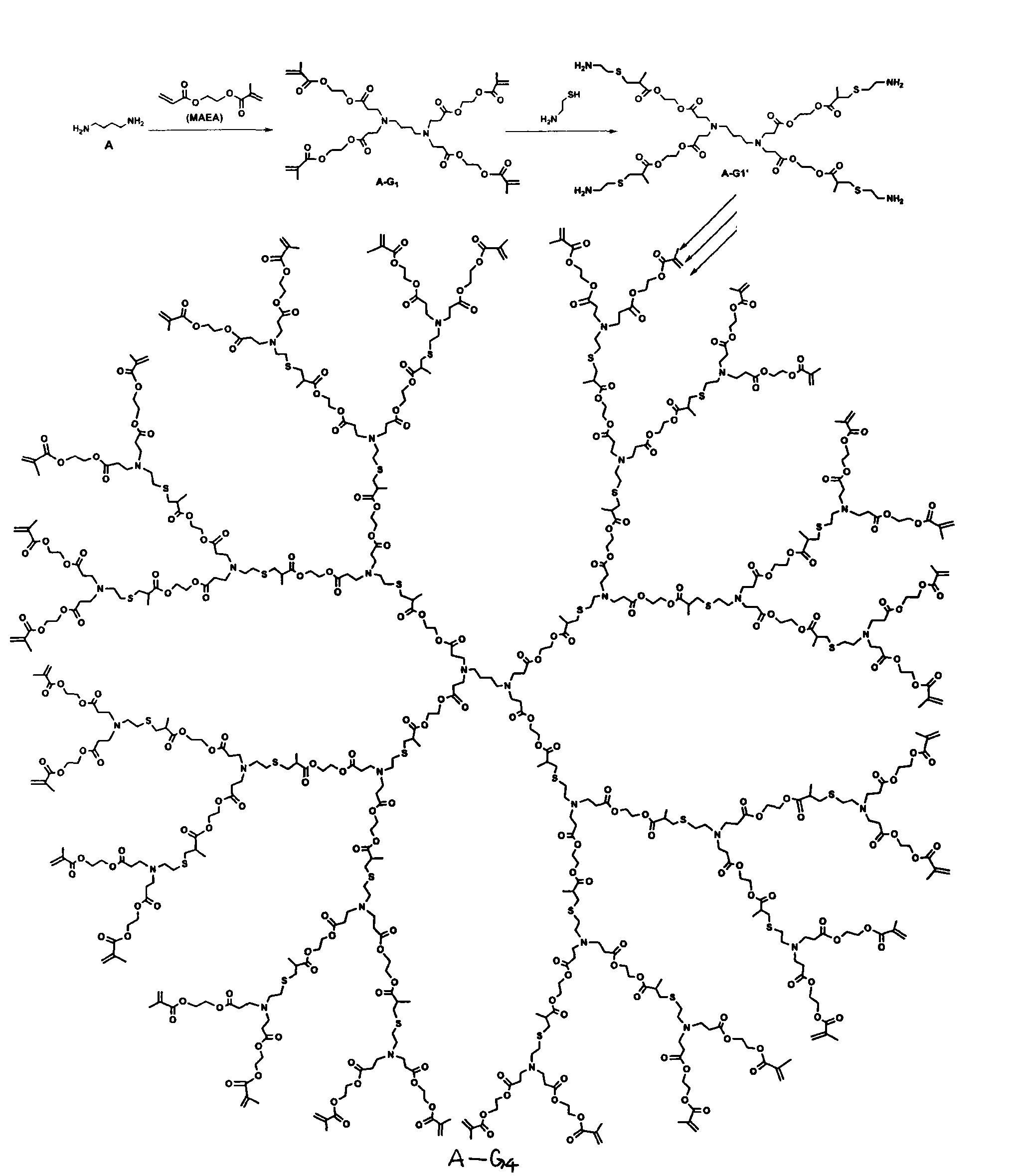
[0030] (2) Synthesis of the first generation polyester dendrimers (A-G 1 )
[0031] Add 0.95g (10.8mmol) of 1,4-butanediamine (A) and 10g (53.9mmol) of MAEA into the flask, stir at room temperature overnight, then react at 70°C for 48h, and wash the reaction solution with n-hexane three times, each time 10ml of n-hexane was dried under vacuum to obtain the produc...
Embodiment 2
[0042] Except adopting ethylenediamine (B) to replace 1,4-butanediamine (A) to react with MAEA in step (2), other operations are the same as in Example 1, and the fourth generation polyester dendrimer B-G is prepared 4 .
Embodiment 3
[0044] Except adopting 1,6-hexamethylenediamine (C) to replace 1,4-butanediamine (A) to react with MAEA in step (2), other operations are the same as in Example 1, and the fourth-generation polyester dendrimer is prepared Shaped macromolecule C-G 4 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com