Catalyst for preparing dimethyl carbonate by using urea alcoholysis and preparation method thereof
A technology of dimethyl carbonate and catalyst, which is applied in the field of urea alcoholysis to prepare dimethyl carbonate catalyst and its preparation, can solve the problems of low reaction conversion rate, high price, product separation, etc., achieve simple preparation method and solve recovery difficulties , the effect of improving stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
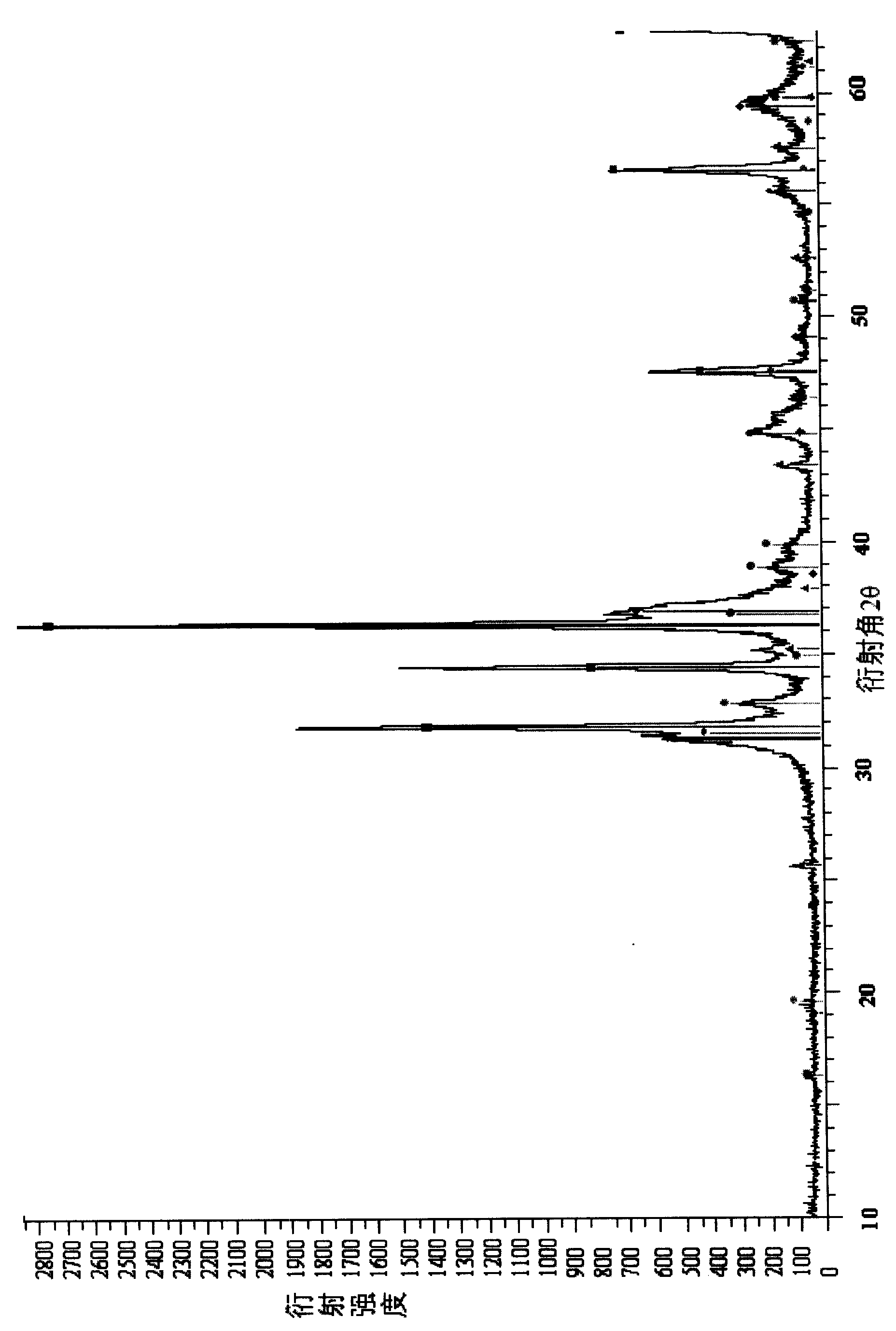
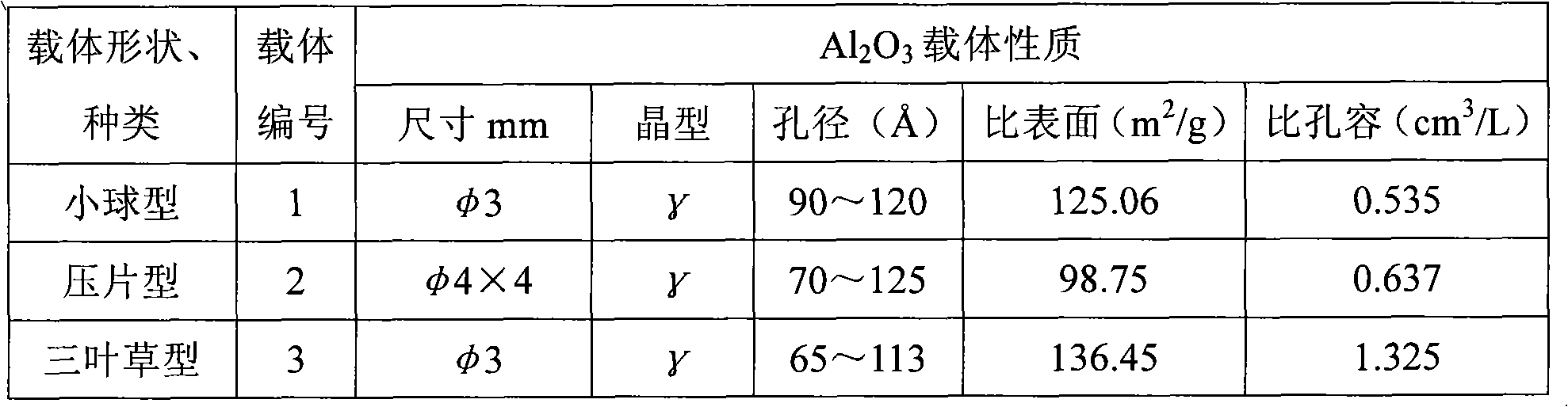
[0053] [Example 1] prepare 85wt% Zn(NO 3 ) 2 Solution 70ml, add the surfactant 1 of 0.05ml / l, adjust pH value 3.0. The Zn of 50wt% (NO 3 ) 2 Solution 50ml, add 0.1ml / l surfactant 2, adjust the pH value to 3.0. At room temperature, 60wt% Zn(NO 3 ) 2 The solution was impregnated onto 50 g of carrier 1, and impregnated at 60° C. for 2 hours. Raise the temperature from 80°C to 150°C at a constant speed and dry for 10 hours. 5 hours from room temperature to 500 ° C, constant temperature roasting for 3 hours. One-step impregnated catalyst with 50 wt% Zn(NO) 2 The solution was impregnated twice, dried and calcined at a constant temperature of 800°C for 3 hours. The composition of the obtained catalyst is 43.2wt% of zinc oxide and 56.8wt% of aluminum oxide, and the content distribution of the active components of the catalyst is shown in Table 5.
[0054] Table 5 Content distribution of catalyst active components
[0055] Location
[0056] Add 300ml of methanol an...
Embodiment 2
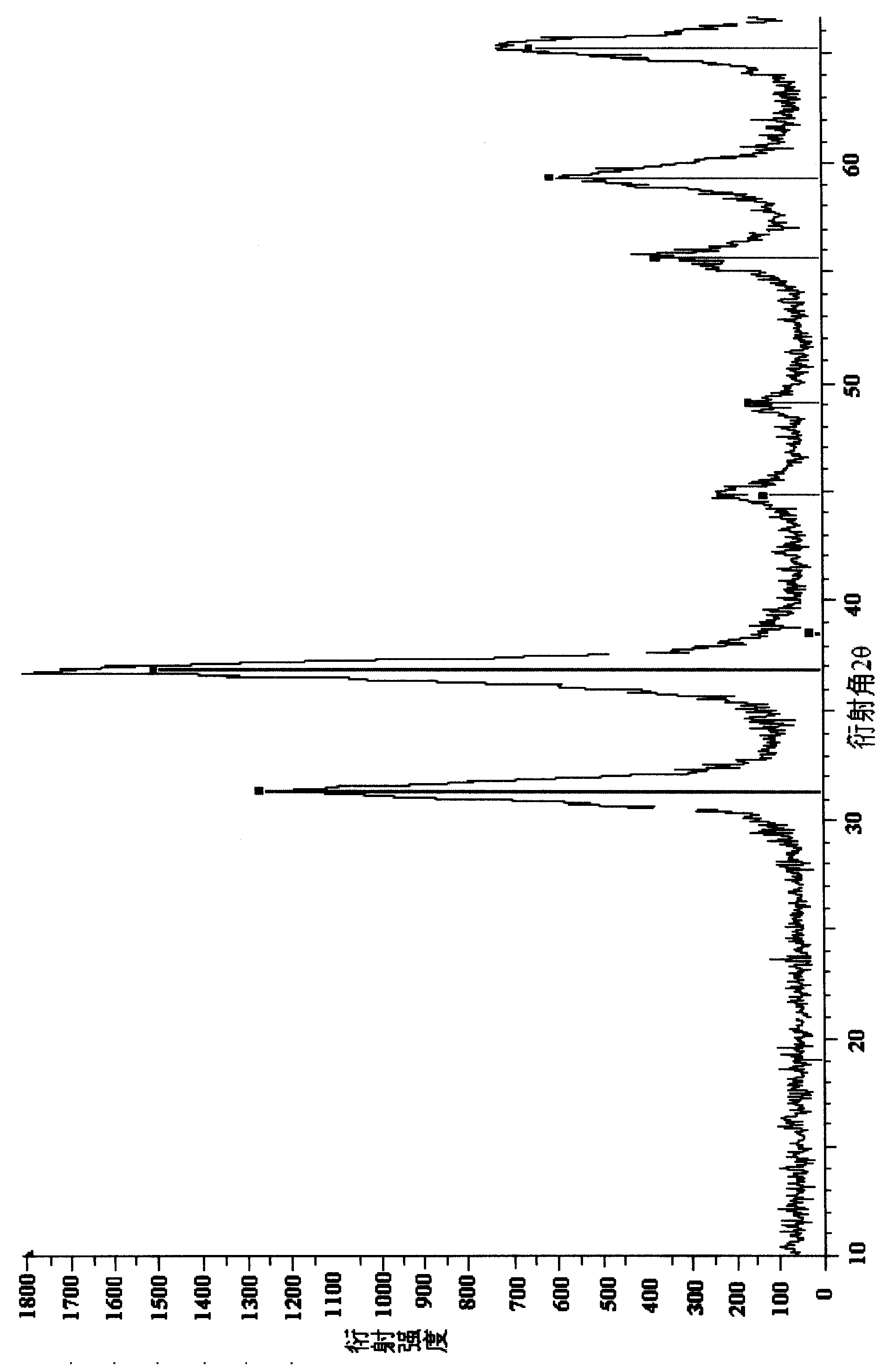
[0057] [embodiment 2] prepare respectively the Pb of 70wt% (NO 3 ) 2 Solution 50ml, add the surfactant 2 of 0.05ml / l, 40wt%Pb (NO3 ) 2 Solution 50ml, add 0.06ml / l surfactant 1, adjust the pH value to 3.0. 60wt% Pb(NO 3 ) 2 The solution was impregnated onto 50 g of carrier 2, and impregnated at a constant temperature of 60° C. for 2 hours. Raise the temperature from 80°C to 150°C at a constant speed and dry for 10 hours. Rising from room temperature to 500°C in 5 hours, and calcination at constant temperature for 5 hours. One-step impregnation of the catalyst with 40 wt% Pb(NO 3 ) 2 The solution was impregnated twice, dried, and baked at a constant temperature of 800° C. for 7 hours. The composition of the obtained catalyst is 31.2wt% of lead oxide and 68.8wt% of aluminum oxide, and the content distribution of the active components of the catalyst is shown in Table 6. Reaction in the autoclave, catalyst addition 16.8g, other conditions are with embodiment 1. The resul...
Embodiment 3
[0060] [Example 3] prepare 60wt% Ca(NO 3 ) 2 Solution 75ml, add the surfactant 3 of 0.03ml / l, 40wt% Ca (NO 3 ) 2 Solution 50ml, add 0.04ml / l surfactant 3, all adjust the pH value to 4.0. 60wt% Ca(NO 3 ) 2 The solution was impregnated onto 50 g of carrier 3, and impregnated at a constant temperature of 60° C. for 2 hours. Raise the temperature from 80°C to 150°C at a constant speed and dry for 10 hours. Rise from room temperature to 500°C in 5 hours, and roast at constant temperature for 3.5 hours. One-step impregnated catalyst with 40wt% Ca(NO 3 ) 2 The solution was impregnated twice, dried, and baked at a constant temperature of 800° C. for 7 hours. The composition of the catalyst is 29.6wt% of calcium oxide and 70.4wt% of alumina, and the content distribution of the active components of the catalyst is shown in Table 7. Reaction in the autoclave, catalyst addition 18.8g, other conditions are with embodiment 1. The results are shown in Table 19.
[0061] Table 7 C...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com