Phase variation electrolyte as well as preparation method and application thereof
An electrolyte and phase transition technology, applied in the field of materials, can solve the problems of lithium storage capacity attenuation, thermal instability, energy loss, etc., and achieve the effects of low self-discharge rate, high self-discharge rate, and reduced impedance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Put the dry beaker of LBOB and acetamide into the glove box after drying. The water content is less than 1ppm. Weigh 20g of LBOB and 80g of acetamide in the beaker, slowly heat to 80°C to melt, and then stir with a magnetic stirrer for 1 -5 hours, it becomes a transparent liquid, and after natural cooling, it becomes a white waxy solid to obtain the required phase transition electrolyte. Its thermal properties were measured with a thermogravimetric analyzer NETSCHSTA 449C. The melting point was 50°C, and the melting point was the phase transition temperature point.
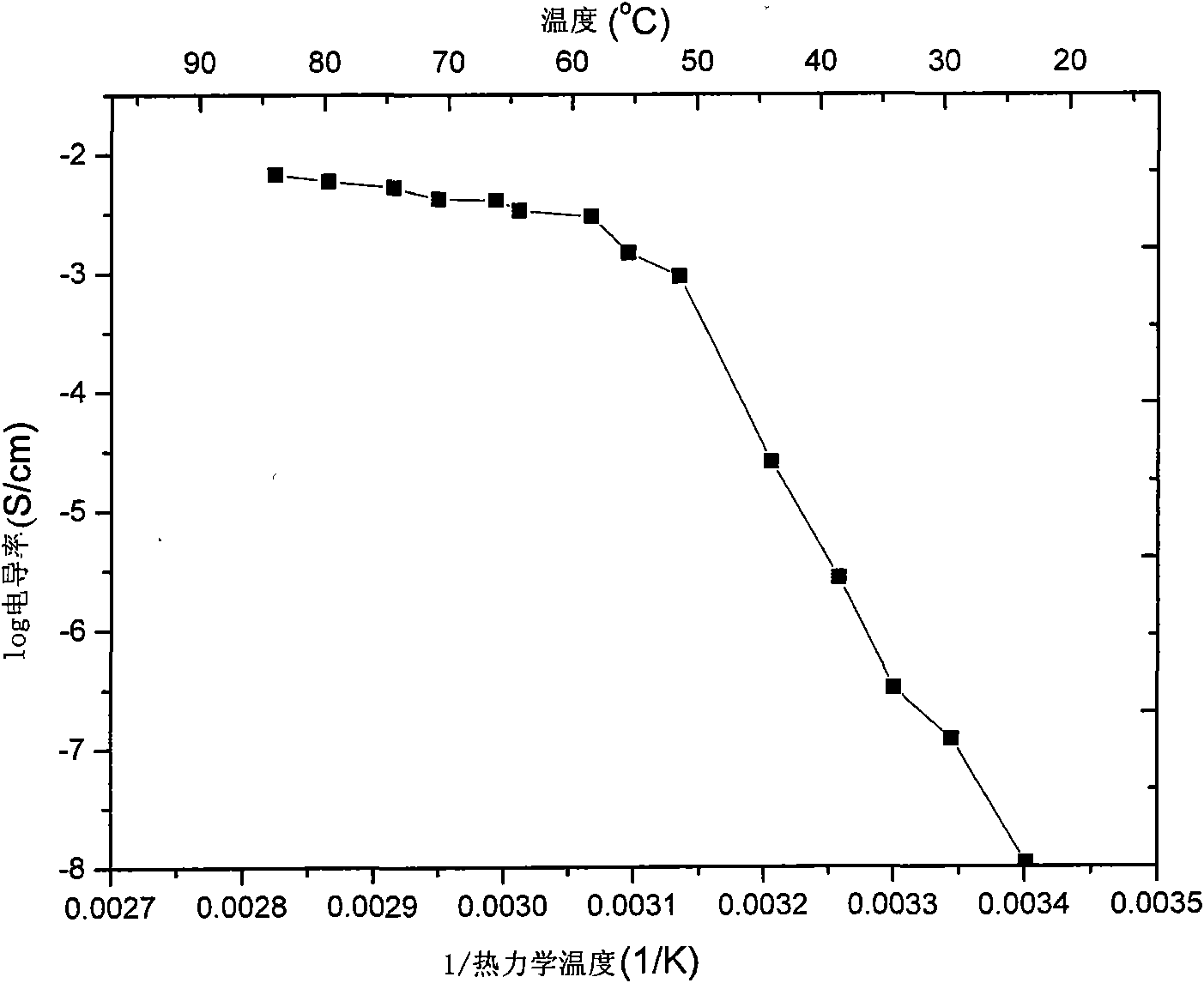
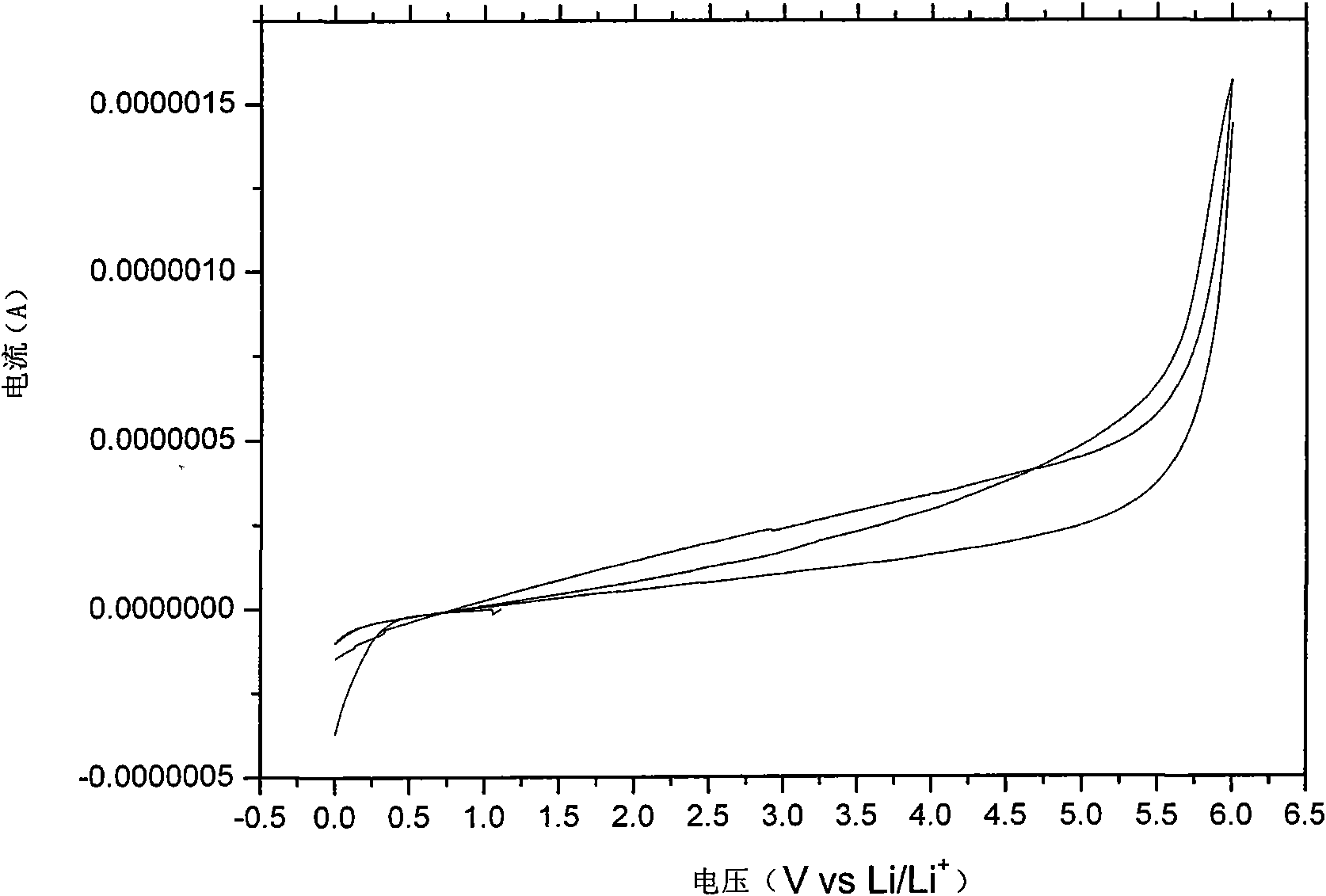
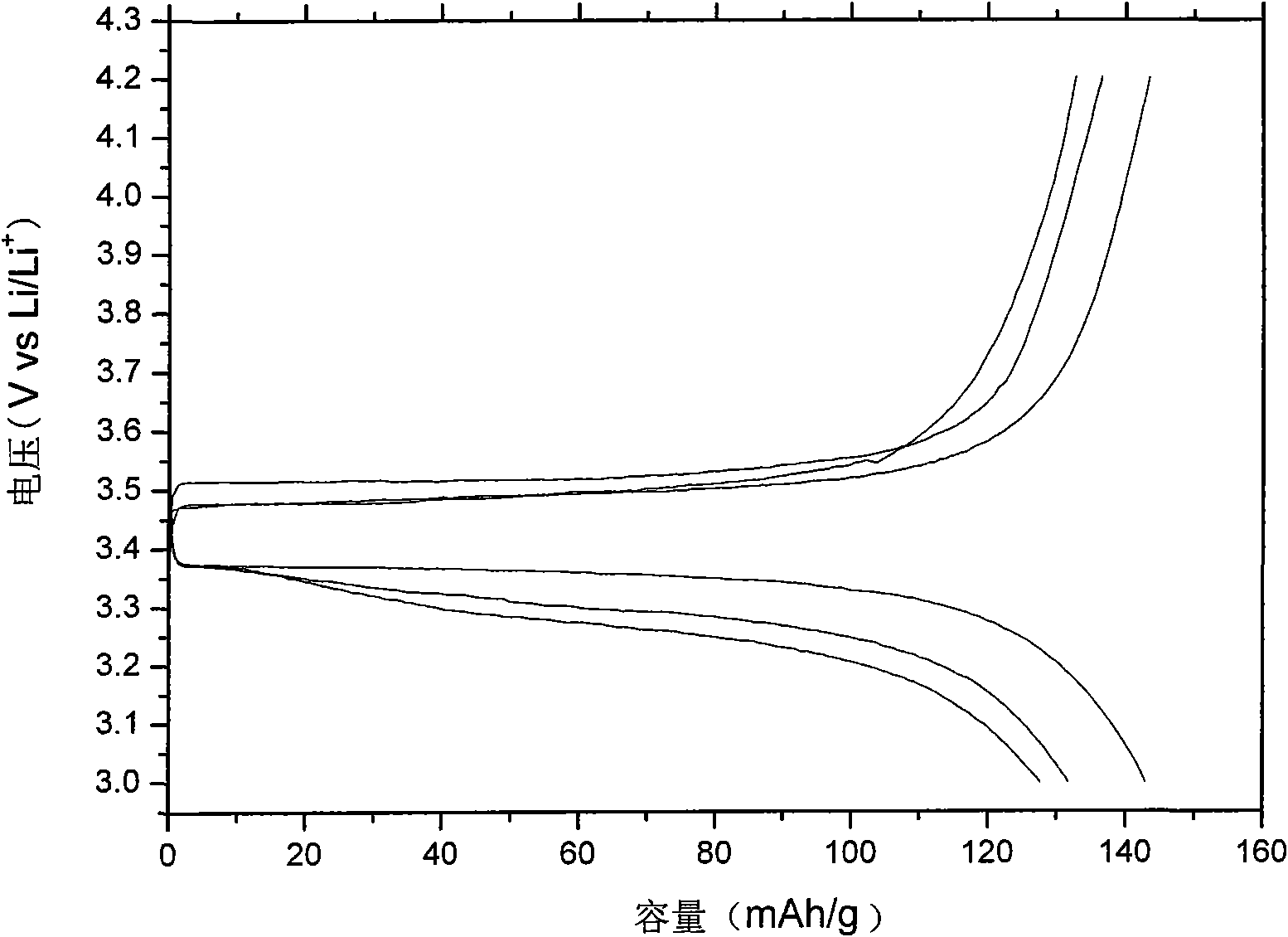
[0044] After melting the electrolyte, put it into a glass conductivity cell with stainless steel electrodes at both ends, and use HP4192 impedance spectrometer to measure its conductivity in the range of 5Hz-13MHz. The measured conductivity at 25°C is 5×10 -5 ms / cm, and the conductivity reaches 4.1mS / cm at 60°C. Used in conjunction with GDW6005 high and low temperature test chamber to measure the conductiv...
Embodiment 2
[0052] Dry LBOB and urea and put them in a glove box (water content is less than 1ppm), weigh 10g LiBOB and 90g urea in a beaker, heat slowly to 100°C to melt, and then stir for 1-5 hours with a magnetic stirrer. It becomes a transparent liquid, and after natural cooling, it becomes a white waxy solid to obtain the required phase transition electrolyte. Its thermal properties were measured by NETSCH STA 449C thermogravimetric analyzer, the melting point was 89°C, and the melting point was the phase transition temperature point.
[0053] After melting the electrolyte, put it into a glass conductivity cell with stainless steel electrodes at both ends, and use HP4192 impedance spectrometer to measure its conductivity in the range of 5Hz-13MHz. The measured conductivity at 25°C is 7×10 -5 ms / cm, reached 2.2mS / cm at 60°C. Used in conjunction with GDW6005 high and low temperature test chamber to measure the conductivity of samples at different temperatures. Using CHI627C electroch...
Embodiment 3
[0056] LBOB, dimethylformamide, SiO 2 and polyacrylonitrile (PAN) put into the glove box after drying (water content is less than 1ppm), weigh 10g LiBOB, 60g dimethylformamide, 10g SiO 2 Put 20g of PAN in a beaker, slowly heat it to 100°C to melt it, then stir it with a magnetic stirrer for 1-5 hours, it becomes a transparent liquid, and after natural cooling, it becomes a white waxy solid to obtain the required phase transition electrolyte. The thermal properties were measured by NETSCH STA 449C thermogravimetric analyzer, the melting point was 54°C, and the melting point was the phase transition temperature point.
[0057] After melting the electrolyte, put it into a glass conductivity cell with stainless steel electrodes at both ends, and use HP4192 impedance spectrometer to measure its conductivity in the range of 5Hz-13MHz. The measured conductivity at 25°C is 4×10 -5 ms / cm, and reached 1.0 mS / cm at 60°C. Used in conjunction with GDW6005 high and low temperature test ch...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| electrical conductivity | aaaaa | aaaaa |
| electrical conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com