Maleic acid cinepazide liposome injection and new application thereof
A technology of cinepazide maleate and cinepazide lipid, which is applied in the field of liposome preparations to achieve the effects of reducing drug toxicity, leakage, and drug side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Example 1 Preparation of cinepazide maleate liposomes
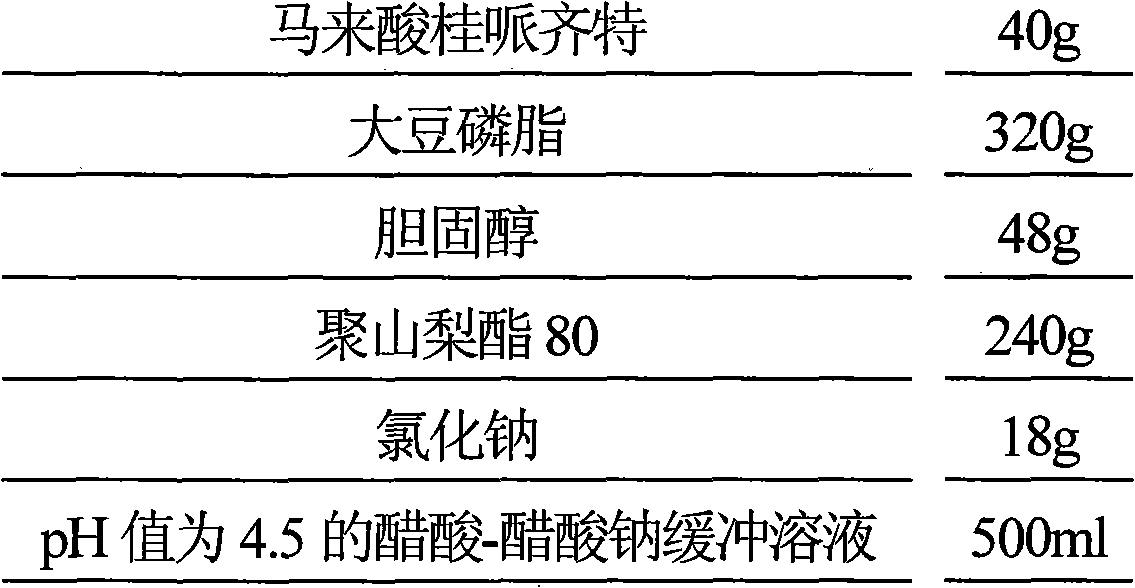
[0042] Prescription (1000 sticks):
[0043]
[0044] Preparation Process
[0045] (1) Dissolve 320g of soybean lecithin, 48g of cholesterol and 240g of polysorbate 80 in 2000ml of n-butanol, slowly inject 2000ml of 0.03mol / L ammonium sulfate solution under stirring, heat and stir to remove n-butanol, put it in an ice bath and ultrasonically 20min, get blank liposome;
[0046] (2) Blank liposome is placed in dialysis bag, seals, and dialysis bag is placed in 0.9% sodium chloride aqueous solution 1500ml and dialyzes 22 hours, removes the ammonium sulfate in liposome external phase;
[0047] (3) Dissolve 40g of cinepazide maleate in 800ml of water, heat the dialyzed blank liposome at 60°C, slowly add the aqueous solution of cinepazide maleate under stirring, continue to keep warm for 20min, add 0.9 500ml of aqueous sodium chloride solution and 500ml of acetic acid-sodium acetate buffer solution with a pH valu...
Embodiment 2
[0051] Example 2 Preparation of cinepazide maleate liposomes
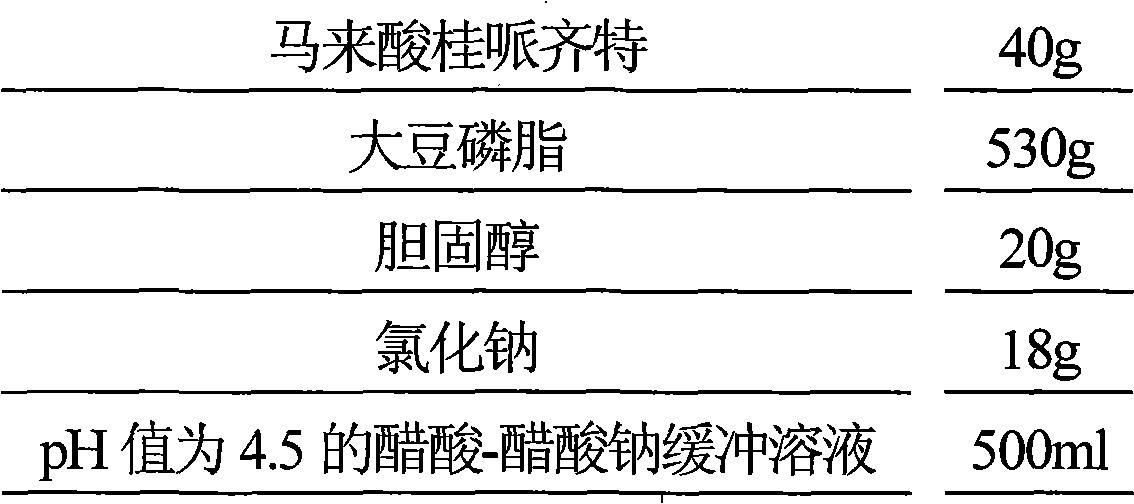
[0052] Prescription (1000 sticks):
[0053]
[0054] Preparation Process
[0055] (1) Dissolve 480g of hydrogenated soybean lecithin, 400g of cholesterol and 320g of polysorbate 80 in 3000ml of benzyl alcohol, slowly inject 3000ml of 0.1mol / L ammonium sulfate solution under stirring, heat and stir to remove benzyl alcohol, put it in an ice bath and ultrasonically for 20min , to obtain blank liposomes;
[0056] (2) Place the blank liposome in the dialysis bag, seal it, place the dialysis bag in 1500ml of 10% glycerol aqueous solution and dialyze for 22 hours, remove the ammonium sulfate in the liposome external phase;
[0057] (3) Dissolve 80g of cinepazide maleate in 1000ml of water, heat the dialyzed blank liposome at 60°C, slowly add the aqueous solution of cinepazide maleate under stirring, continue to keep warm for 30min, add 10 500ml of aqueous solution of % glycerol and 500ml of potassium dihydrogen...
Embodiment 3
[0065] Example 3 Preparation of cinepazide maleate liposomes
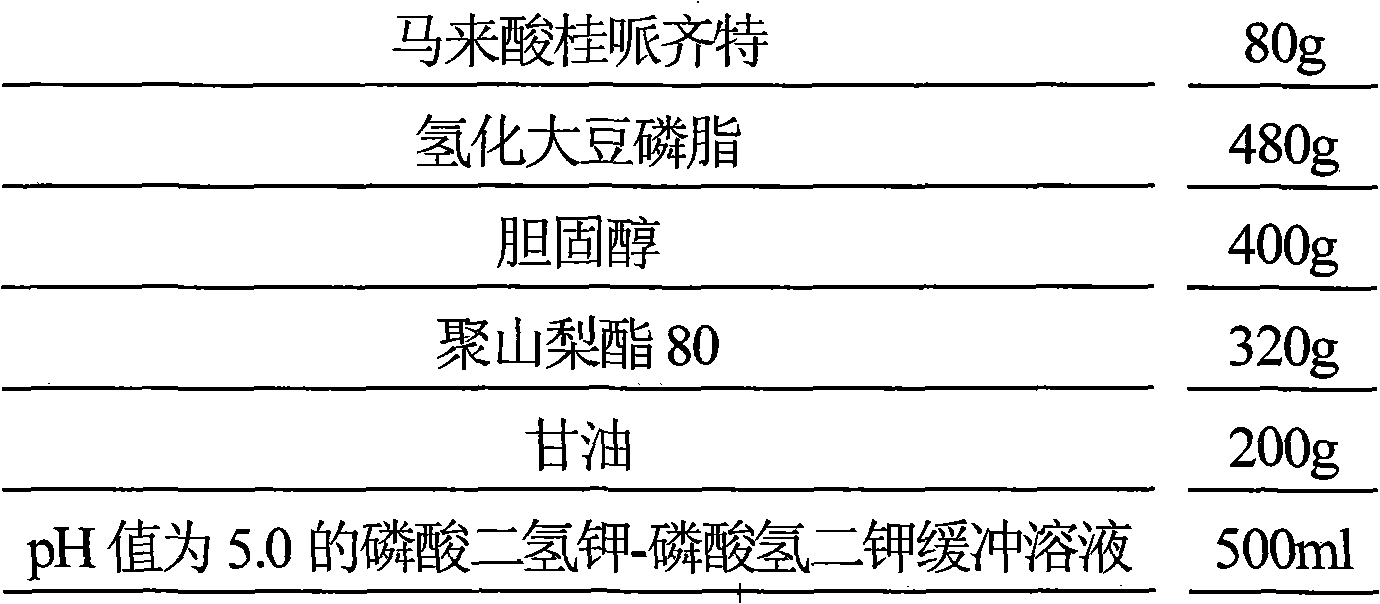
[0066] Prescription (1000 sticks):
[0067]
[0068] Preparation Process
[0069] (1) Dissolve 1600g dioleoylphosphatidylcholine, 640g cholesterol and 960g polysorbate 80 in 6000ml isopropanol, slowly inject 6000ml of 0.5mol / L ammonium sulfate solution under stirring, heat and stir to evaporate isopropanol, Place in an ice bath and sonicate for 10 minutes to obtain blank liposomes;
[0070] (2) Blank liposome is placed in the dialysis bag, seals, and the dialysis bag is placed in 5% glucose aqueous solution 2250ml and dialyzes for 22 hours, removes the ammonium sulfate in the liposome external phase;
[0071] (3) Dissolve 320g of cinepazide maleate in 1500ml of water, heat the dialyzed blank liposome at 60°C, slowly add the aqueous solution of cinepazide maleate under stirring, continue to keep warm for 20min, add 5 750ml of aqueous glucose solution and 500ml of citric acid-sodium citrate buffer solution ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com