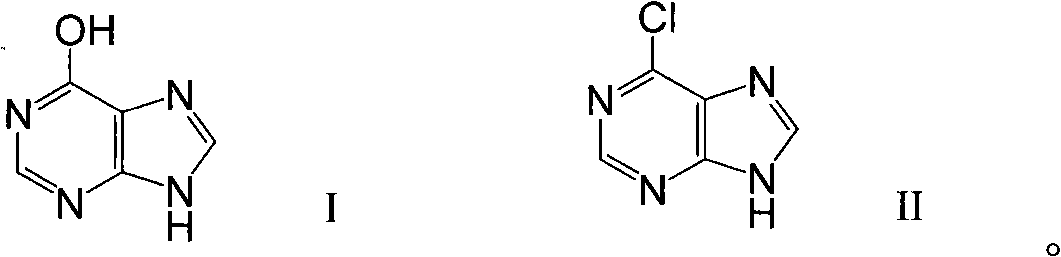
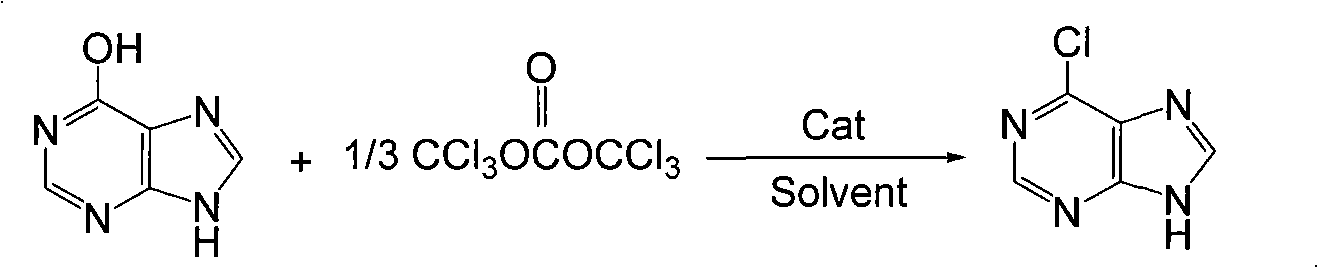Synthesis method of 6-chloropurine
A synthetic method and technology of chloropurine, applied in chemical instruments and methods, organic compound/hydride/coordination complex catalysts, organic chemistry, etc., can solve problems such as difficult and accurate measurement, pollution, equipment corrosion, etc., and achieve the elimination of safety Large hidden dangers, simple and safe operation, avoiding high cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] The amount ratio hypoxanthine of feeding material: two (trichloromethyl) carbonate: organic amine catalyst is 1: 0.36: 0.2, and hypoxanthine feeding amount is 2.74g (0.02mol), two (trichloromethyl) carbonic acid The ester charging amount is 2.16g (0.0072mol), the organic amine catalyst is triethylamine, the consumption is 0.41g (0.004mol), the organic solvent is toluene, and the consumption is 10 times (27.4g) of hypoxanthine quality.
[0018] First, triethylamine was dissolved in 10g of toluene, cooled in an ice bath, and the toluene solution of bis(trichloromethyl)carbonate was slowly added dropwise (2.16g of bis(trichloromethyl)carbonate was dissolved in 17.4g of toluene), Control the rate of addition to keep the temperature of the reaction solution at 0~5°C. After the dropwise addition, continue to stir for half an hour; add hypoxanthine and continue to stir, and keep the reaction at 0~5°C for 3 hours; remove the ice bath, and stir for half an hour at room temperatur...
Embodiment 2
[0021] The amount ratio hypoxanthine of feeding material: two (trichloromethyl) carbonate: organic amine catalyst is 1: 0.5: 0.3, and hypoxanthine feeding amount is 2.74g (0.02mol), two (trichloromethyl) carbonic acid The ester charging amount is 3.0g (0.01mol), the organic amine catalyst is N-methylpyrrole, the consumption is 0.48g (0.006mol), the organic solvent is xylene, and the consumption is 10 times (27.4g) of hypoxanthine quality.
[0022] First dissolve pyridine in 10g of toluene, cool in an ice bath, slowly add a toluene solution of bis(trichloromethyl)carbonate (3.0g of bis(trichloromethyl)carbonate dissolved in 17.4g of toluene) dropwise, and drop Acceleration keeps the temperature of the reaction solution at 0-5°C. After the dropwise addition, continue to stir for half an hour; add hypoxanthine, and maintain the reaction at 0-5°C for 2 hours; remove the ice bath, stir and react at room temperature for half an hour, and then heat up to 110 DEG C (to reflux) reactio...
Embodiment 3
[0024]The amount ratio hypoxanthine of feed material: bis(trichloromethyl) carbonate: organic amine catalyst is 1: 0.4: 0.4, hypoxanthine feeding amount is 2.74g (0.02mol), bis(trichloromethyl) carbonic acid The ester charging amount is 2.4g (0.008mol), the organic amine catalyst is N-methylpyrrole, the consumption is 0.65g (0.008mol), the organic solvent is chlorobenzene, and the consumption is 20 times (54.8g) of hypoxanthine quality.
[0025] First dissolve N-methylpyrrole in 20g of chlorobenzene, cool in an ice bath, and slowly add a chlorobenzene solution of bis(trichloromethyl)carbonate (2.4g of bis(trichloromethyl)carbonate dissolved in 34.8 g chlorobenzene), control the rate of addition to keep the temperature of the reaction solution at 0~5°C, after the dropwise addition, continue to stir for half an hour; put in hypoxanthine, and keep the reaction at 0~5°C for 4 hours; remove the ice bath, and stir at room temperature React for half an hour, then heat up to 132°C (to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com