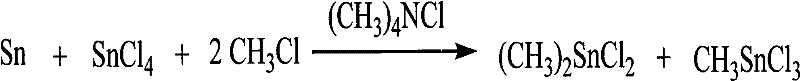Method for preparing oxidative methyl tin
A technology of methyl tin oxide and tin tetrachloride, which is applied in the direction of tin organic compounds, can solve the problems of incomplete reaction, high reactivity, and low utilization rate of tin raw materials, so as to reduce reaction time, increase contact area, and improve Effect of Reaction Utilization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0015] The method for preparing methyl tin oxide provided by the present invention uses metal tin, methyl chloride, tin tetrachloride and ammonia water as raw materials, including: a, alkane halogenation reaction of tin; b, stable reaction of the first mixed intermediate; c, The distillation preparation of the second mixed intermediate; d, the oxidation reaction of the third mixed intermediate.
[0016] a. Alkyl halogenation reaction of tin
[0017] The alkane halogenation reaction of tin can be represented by the following chemical equation:
[0018]
[0019] There are often some side reactions in the alkane halogenation reaction of tin, mainly including:
[0020] (CH 3 ) 2 SnCl 2 +Sn+2CH 3 Cl→(CH 3 ) 3 SnCl+SnCl 2
[0021] b, the stable reaction of the first mixed intermediate
[0022] The stabilization reaction can be represented by the following chemical equation:
[0023] (CH 3 ) 3 SnCl+SnCl 4 →(CH 3 ) 2 SnCl 2 +CH 3 SnCl 3
[0024] d, the oxidation...
Embodiment 1
[0029] a. Alkyl halogenation reaction of tin
[0030] Carry out under 130 ℃ and 0.2Mpa condition, reaction time is 1.5h and makes the first mixed intermediate, the catalyzer of this reaction is trimethylamine, and the mass ratio of charging of metal tin, methyl chloride and tin tetrachloride is 50: 40: 10. The dosage of catalyst is 1% of the mass of tin metal.
[0031] Mix metal tin and trimethylamine in advance, and heat it to melt, and then mix the mixture with methyl chloride and tin tetrachloride in the form of mist, which greatly increases the contact area between tin and methyl chloride, and makes the reaction as possible as possible. The formation of dimethyl tin dichloride greatly reduces the formation of trimethyl tin chloride, also improves the reaction utilization rate of tin and methyl chloride, reduces the reaction time, and wherein the utilization rate of tin reaches more than 99%, without The methyl chloride of reaction is reclaimed again, can be used for next ...
Embodiment 2
[0045] a. Alkyl halogenation reaction of tin
[0046] Carried out under the conditions of 140°C and 0.3Mpa, the reaction time is 2h to obtain the first mixed intermediate, the catalyst for this reaction is trimethylamine, and the mass ratio of metal tin, methyl chloride and tin tetrachloride is 40:40:10 , The feed amount of the catalyst is one percent of the mass of tin metal.
[0047] Mix metal tin and trimethylamine in advance, and heat it to melt, and then mix the mixture with methyl chloride and tin tetrachloride in the form of mist, which greatly increases the contact area between tin and methyl chloride, and makes the reaction as possible as possible. The formation of dimethyl tin dichloride greatly reduces the formation of trimethyl tin chloride, also improves the reaction utilization rate of tin and methyl chloride, reduces the reaction time, and wherein the utilization rate of tin reaches more than 99%, without The reacted methyl chloride is reclaimed and can be used...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com