Coumarin compound acidulated by glucal and application thereof
A technology of glucuronic acid and coumarin, which is applied in the field of medicine and can solve the problem of anti-cancer activity reports only targeting
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] Embodiment 1 preparation compound GLU002 and GLU003
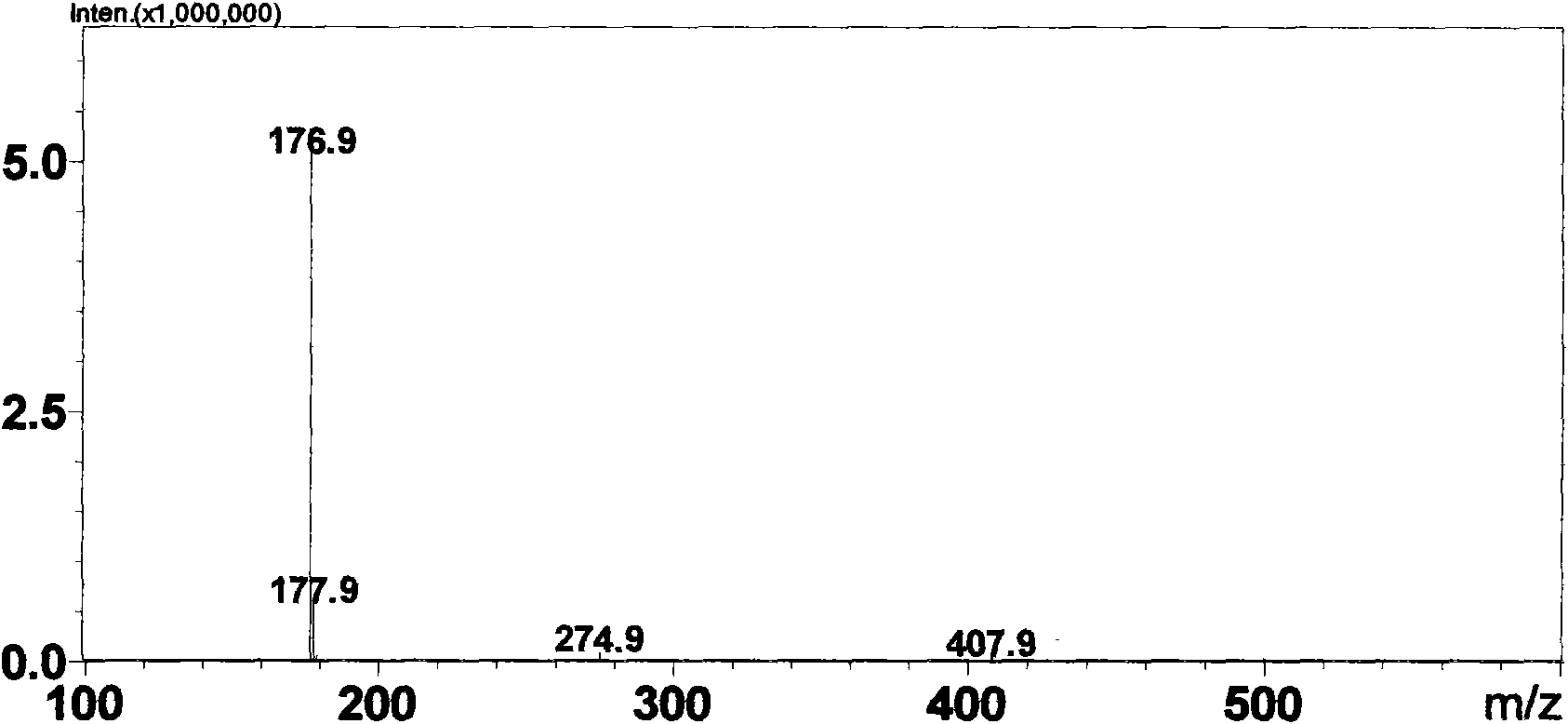
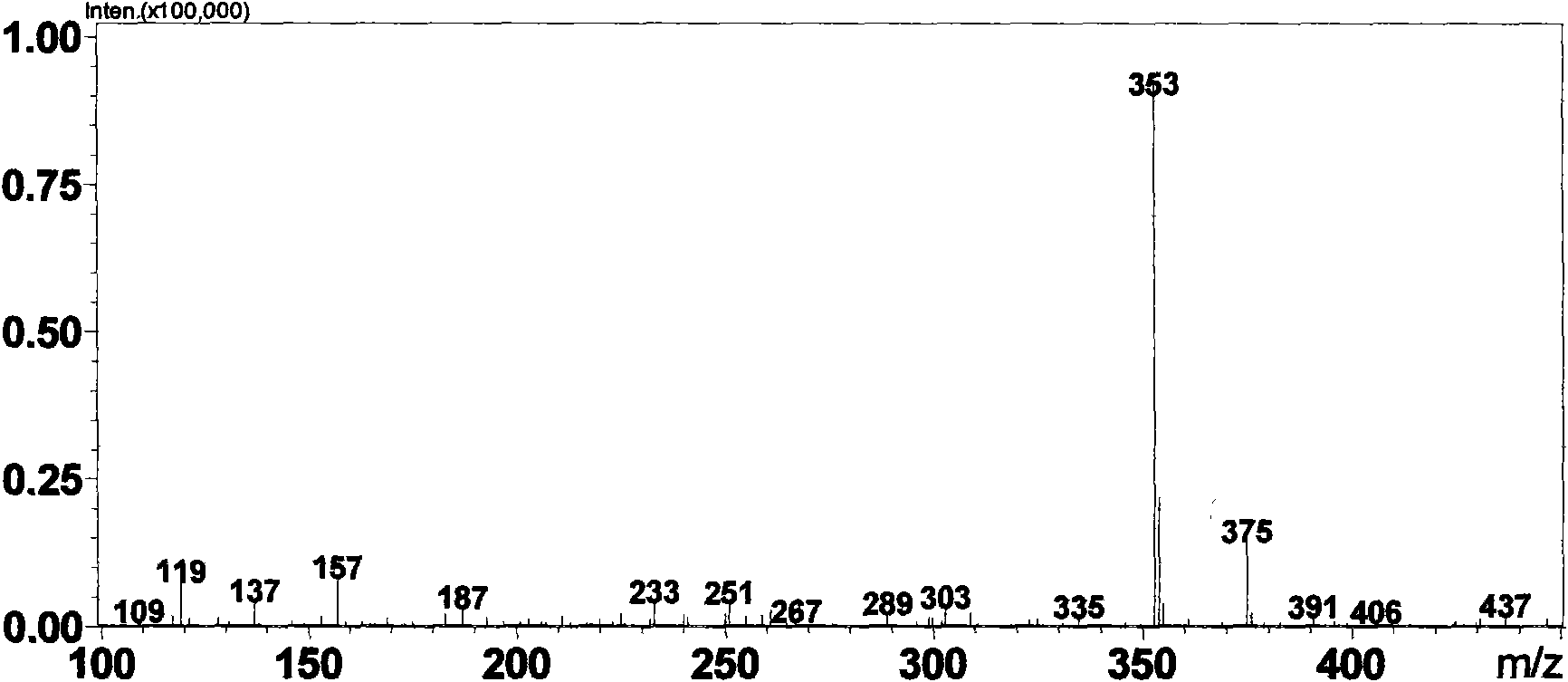
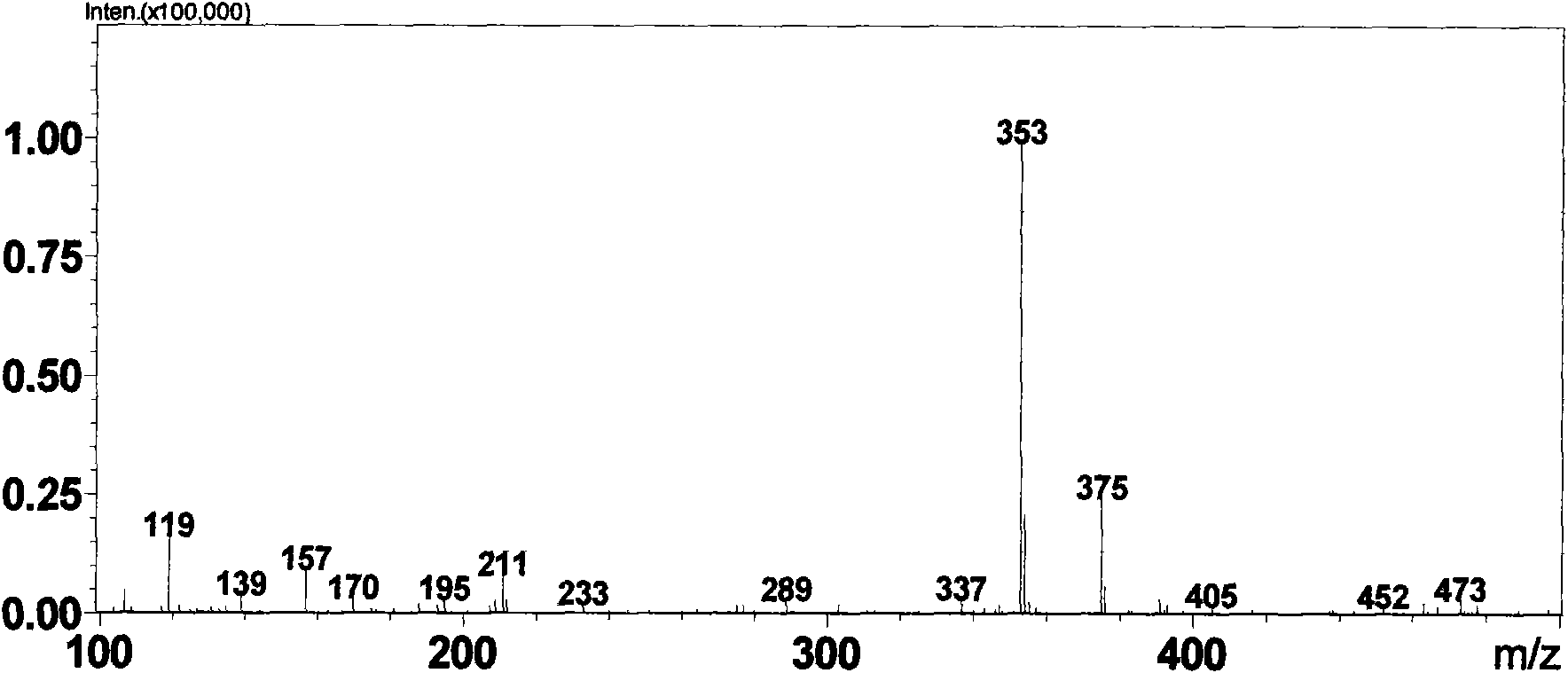
[0064] Weigh 1g daphnetin (7,8-dihydroxycoumarin) sample (measured by HPLC external standard method, content is 99%, LC-MS spectrogram see figure 1 ) was dissolved in 1ml of methanol system, added to the 100ml reaction system of potassium phosphate buffer (pH 7.4) containing rat liver microsomes (0.5mg / m1) and UDPGA (5μM) and fully reacted for 2h, HPLC detection showed daphnetin conversion completely. After centrifugation, the reaction solution was rotary evaporated to 10ml, and passed through a reverse phase (YMC, C18 column) high-performance liquid chromatography system, using a mobile phase of water: acetonitrile (50:50) to rinse at a flow rate of 1ml / min to obtain 80mg of compound GLU002 (content 98%, LC-MS spectrum see figure 2 ) and 78mg GLU003 (content 95%, LC-MS spectrum see image 3 ). The preparation of other glucuronidated coumarin compounds is similar to the reaction conditions and steps.
Embodiment 2
[0065] Embodiment 2 Glucuronidated coumarin compound anti-arthritis experiment
[0066] Twenty-one SD rats with a body weight of about 200 g were injected into the right paw with 0.1 ml of Freund's complete adjuvant, and were randomly divided into 3 groups after 7 days, that is, group A fed with 50 mg / kg of glucuronidated coumarin compounds every day, Group B fed distilled water daily. In addition, 7 SD rats without any treatment were set as the normal control group C. After 21 days, the size of the swelling on the right paw of the rats in each experimental group was measured, and blood was collected from the carotid artery to measure the serum immune indexes. The relevant results are shown in Table 1.
[0067] Table 1 Experimental results of rat arthritis model
[0068]
[0069]
[0070] Mean±S.D., n=7.
[0071] #P<0.05 vs. normal group.
[0072] *P<0.01 vs. model group.
Embodiment 3
[0073] Embodiment 3 glucuronidated coumarin compound antitumor activity experiment
[0074] In vitro anti-tumor activity test: leukemia cells (K562), non-small cell lung cancer cells (A549), colon cancer cells (HT-29), ovarian cancer cells (OVCAR-3), human osteosarcoma cells (Saos-2), Six kinds of tumor cells including breast cancer cells (MCF-7) were used to determine the in vitro antitumor activity of twenty kinds of glucuronidated coumarin compounds. Six kinds of tumor cells were inoculated in 96-well flat-bottomed culture plates respectively, added with sterile culture medium of RPMI 1640+10% NBS+gentamycin, and incubated at 37°C in CO 2 Incubate in an incubator for 24h. After the drug is dissolved with DMSO, it is diluted with a culture medium to a solution with different concentration gradients, and the final concentration of DMSO is lower than 0.1%. After being cultured for 48 h under the action of the test compound, the cell viability was determined by the SRB method...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com