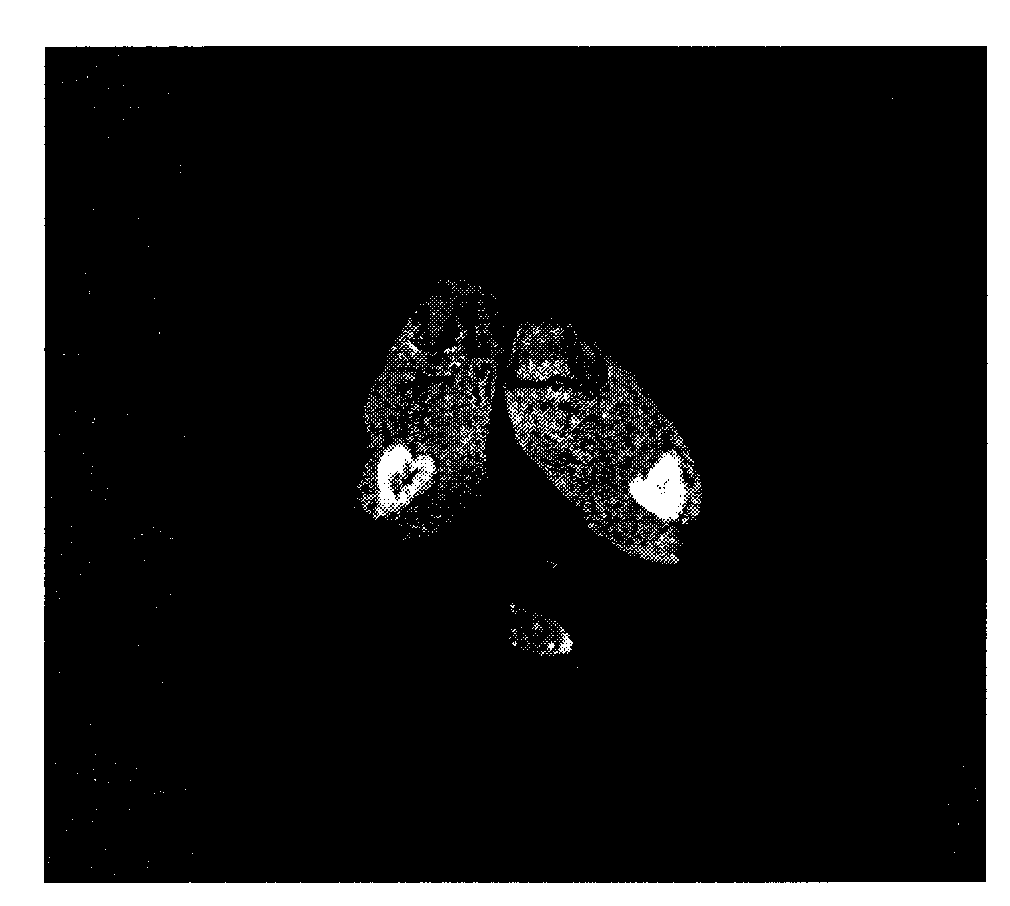
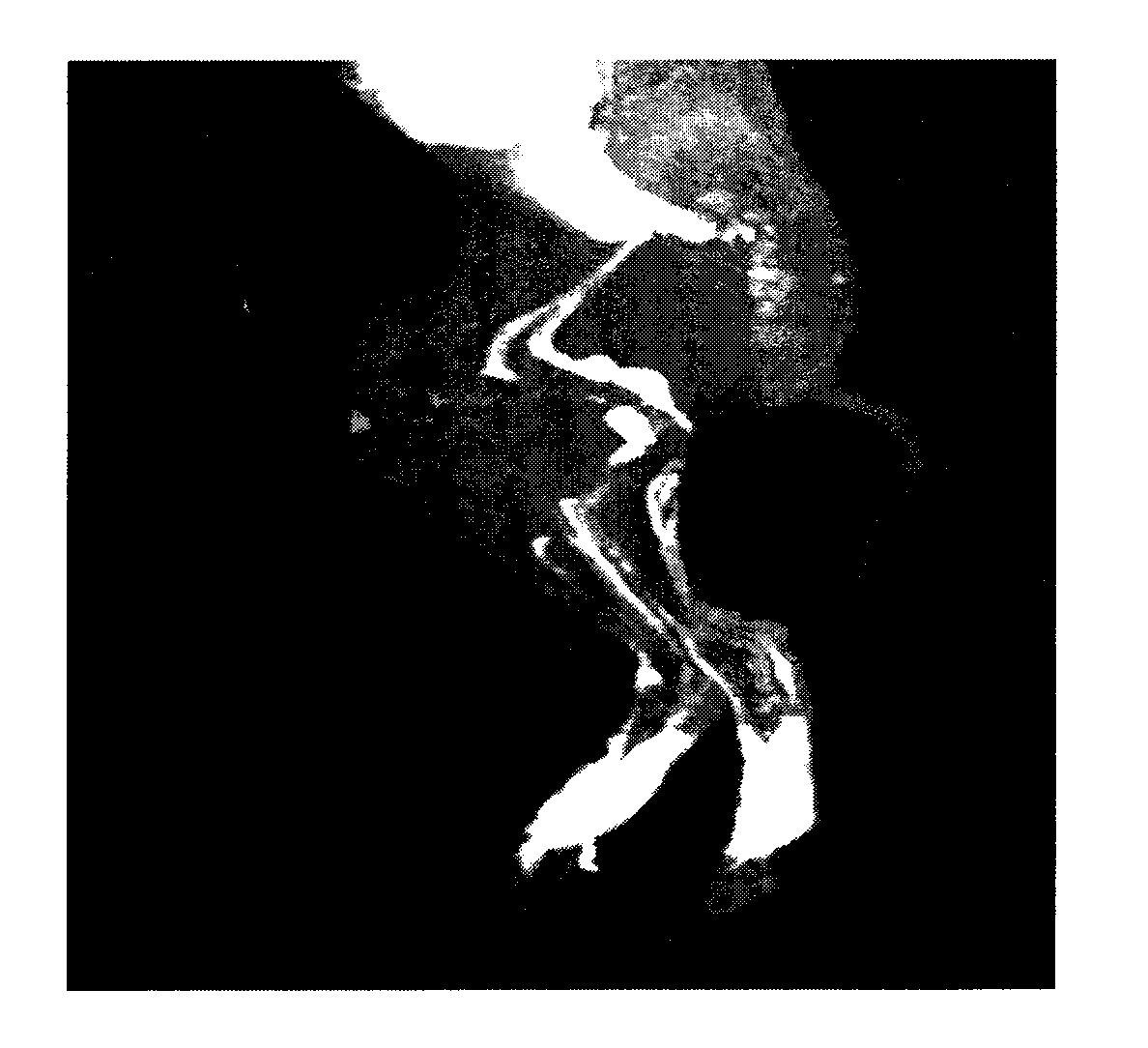Polysaccharide macromolecular paramagnetic metal complex and synthesis method and application thereof
A paramagnetic metal and macromolecular technology, applied in the field of polysaccharide macromolecular paramagnetic metal complexes and their synthesis and application, can solve the problem of lack of tissue or organ selectivity or targeting, and short retention time of Magnevist And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Dissolve 1.62g of dextran (molecular weight: 20,000) and 5mL of triethylamine in 100mL of dimethyl sulfoxide, slowly add 0.98g of bromoethane containing 1, 4, 7, 10 -Azacyclododecanetetraacetic acid DOTA 30ml dimethyl sulfoxide solution, react at room temperature for 4h, rise to 40°C and continue to react for 48h at room temperature, filter, evaporate part of the solvent under reduced pressure, cool to room temperature, and pour the residual solution under stirring into a mixed solvent of 600mL of absolute ethanol-dehydrated ether, the volume ratio of absolute ethanol: absolute ether is 1:2, a pale yellow precipitate is produced, filtered, drained, and the resulting solid is dissolved in distilled water, and dissolved in absolute ethanol , and can also be reprecipitated with anhydrous ether, the light yellow solid was collected and dried in vacuo to obtain 2.21 g of solid with a yield of 85%. 2.21g of the obtained solid was dissolved in 20mL of twice distilled water, an...
Embodiment 2
[0060] 8.08g of agar (molecular weight is 40,000) and 10mL of triethylamine are dissolved in 200mL of dimethyl sulfoxide, at room temperature, under the condition of magnetic stirring, dropwise add the solution containing 3.84g of p-thiocyanate benzyl-DO3A 60ml dimethyl sulfoxide solution, react at room temperature for 4h, rise to 50°C and continue to react for 48h, filter, evaporate part of the solvent under reduced pressure, cool to room temperature, pour the residual solution into 1000mL of anhydrous ethanol-anhydrous ether mixed solvent under stirring In the method, the volume ratio of absolute ethanol: anhydrous ether is 1:2, a light yellow precipitate is produced, filtered, and drained, and the obtained solid is dissolved in distilled water, reprecipitated with absolute ethanol or anhydrous ether, and collected shallow The yellow solid was dried in vacuo to obtain 9.54 g of solid, with a yield of 80%.
[0061] 9.54g of the obtained solid was dissolved in 100mL of twice d...
Embodiment 3
[0063] 1.14g of hyaluronic acid (molecular weight: 80,000) and 5mL of triethylamine were dissolved in 50mL of dimethyl sulfoxide, and at room temperature and under magnetic stirring conditions, 1.47g of diethylenetriaminepentaacetic acid (DTPA) was added in batches under stirring. ) Dimethyl sulfoxide solution of mono-N-hydroxysuccinimide active ester, react at room temperature for 4h, rise to 80°C and continue to react for 48h at room temperature, filter, evaporate part of the solvent under reduced pressure, cool to room temperature, and pour the residual solution under stirring into the mixed solvent of 500mL absolute ethanol-anhydrous ether, the volume ratio of absolute ethanol: absolute ether is 1:2, a light yellow precipitate is produced, filtered, and drained, and the obtained solid is dissolved in distilled water, and then dissolved in absolute ethanol , and can also be reprecipitated with anhydrous ether, the light yellow solid was collected and dried in vacuo to obtain...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com