Method for recycling uranium from uranium extraction coal residue with wet method
A technology for recovering uranium and coal slag, applied in the field of metallurgy, can solve problems such as idle waste of uranium resources, environmental pollution, uranium extraction, etc., and achieve the effects of promoting local economy and society, developing circular economy, and high recovery rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
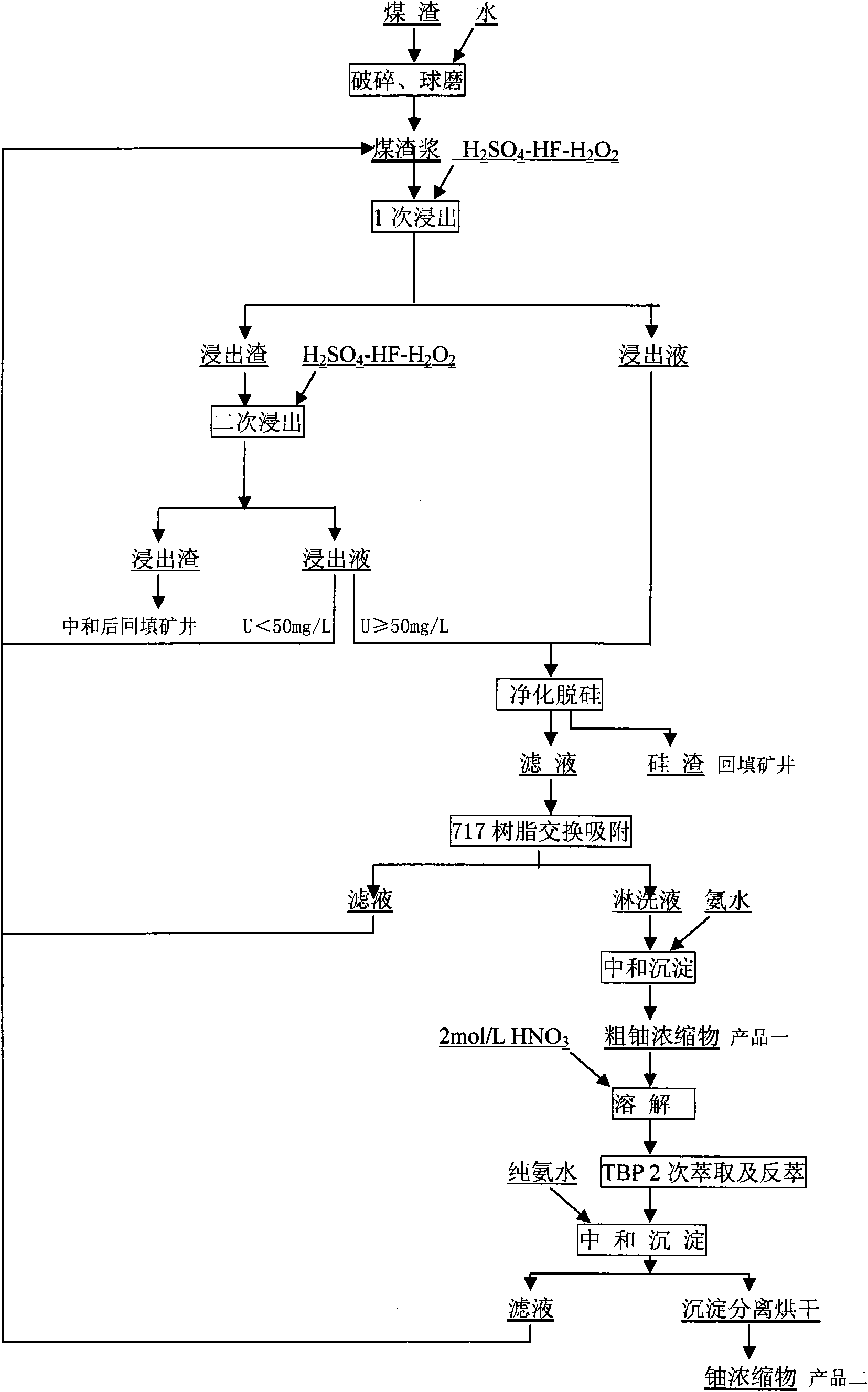
[0020] 1) The cinder is first crushed by a ball mill to below 200 mesh;
[0021] 2) Mixing cinders with a calcium content higher than 6% and cinders with a calcium content lower than 2% and stirring them evenly to reduce the calcium content in the raw cinders to 2-5%, so as to achieve a better leaching effect;
[0022] 3) Take 100g of pulverized coal cinder, add 150mL of 98g / L sulfuric acid solution and 25mL of 5g / L hydrofluoric acid solution, and use 30mL hydrogen peroxide with a concentration of 30% by mass as an oxidizing agent for oxidative leaching 150mL of sulfuric acid solution of 98g / L and 25mL of hydrofluoric acid solution of 5g / L, and 3mL of hydrogen peroxide with a mass percentage concentration of 30% are used as oxidant to carry out oxidative leaching of the leaching residue;
[0023] 4) The primary leaching filtrate of uranium is adjusted to pH 9.8-10.2 with ammonia water with a concentration of 25% by mass, and the silicon in the solution becomes a silicic acid p...
Embodiment 2
[0026] 1) The cinder is first crushed by a ball mill to below 200 mesh;
[0027] 2) Mixing cinders with a calcium content higher than 6% and cinders with a calcium content lower than 2% and stirring them evenly to reduce the calcium content in the raw cinders to 2-5%, so as to achieve a better leaching effect;
[0028] 3) Take 100g of pulverized coal slag, add 100mL of 98g / L sulfuric acid solution and 50mL of 5g / L hydrofluoric acid solution, and use 2.5mL of 30% hydrogen peroxide as an oxidizing agent to oxidize For leaching, use 100 mL of 98 g / L sulfuric acid solution, 50 mL of 5 g / L hydrofluoric acid solution, and 2.5 mL of hydrogen peroxide with a concentration of 30% by mass as the oxidizing agent for oxidative leaching of the leaching residue;
[0029] 4) The primary leaching filtrate of uranium is adjusted to pH 9.8-10.2 with ammonia water with a concentration of 25% by mass, and the silicon in the solution becomes a silicic acid precipitate. Ion exchange resin for exch...
Embodiment 3
[0032] 1) The cinder is first crushed by a ball mill to below 200 mesh;
[0033] 2) Mixing cinders with a calcium content higher than 6% and cinders with a calcium content lower than 2% and stirring them evenly to reduce the calcium content in the raw cinders to 2-5%, so as to achieve a better leaching effect;
[0034] 3) Take 100 g of pulverized coal cinder, add 150 mL of 98 g / L sulfuric acid solution and 50 mL of 5 g / L hydrofluoric acid solution, and use 5 mL of 30% hydrogen peroxide as an oxidizing agent for oxidative leaching , the leaching residue is oxidized and leached again with 150 mL of 98 g / L sulfuric acid solution and 50 mL of 5 g / L hydrofluoric acid solution, and 5 mL of hydrogen peroxide with a mass percent concentration of 30% as an oxidizing agent;
[0035] 4) The primary leaching filtrate of uranium is adjusted to pH 9.8-10.2 with ammonia water with a concentration of 25% by mass, and the silicon in the solution becomes a silicic acid precipitate. Ion exchang...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com