Oral sustained release granules
A technology of drug delivery and granules, which is applied in the direction of pharmaceutical formulas, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., which can solve the problems of unfavorable mass industrial production, incomplete dissolution of drugs, and large one-time investment, etc. problems, to achieve the effects of easy control of production quality, strong drug activity, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
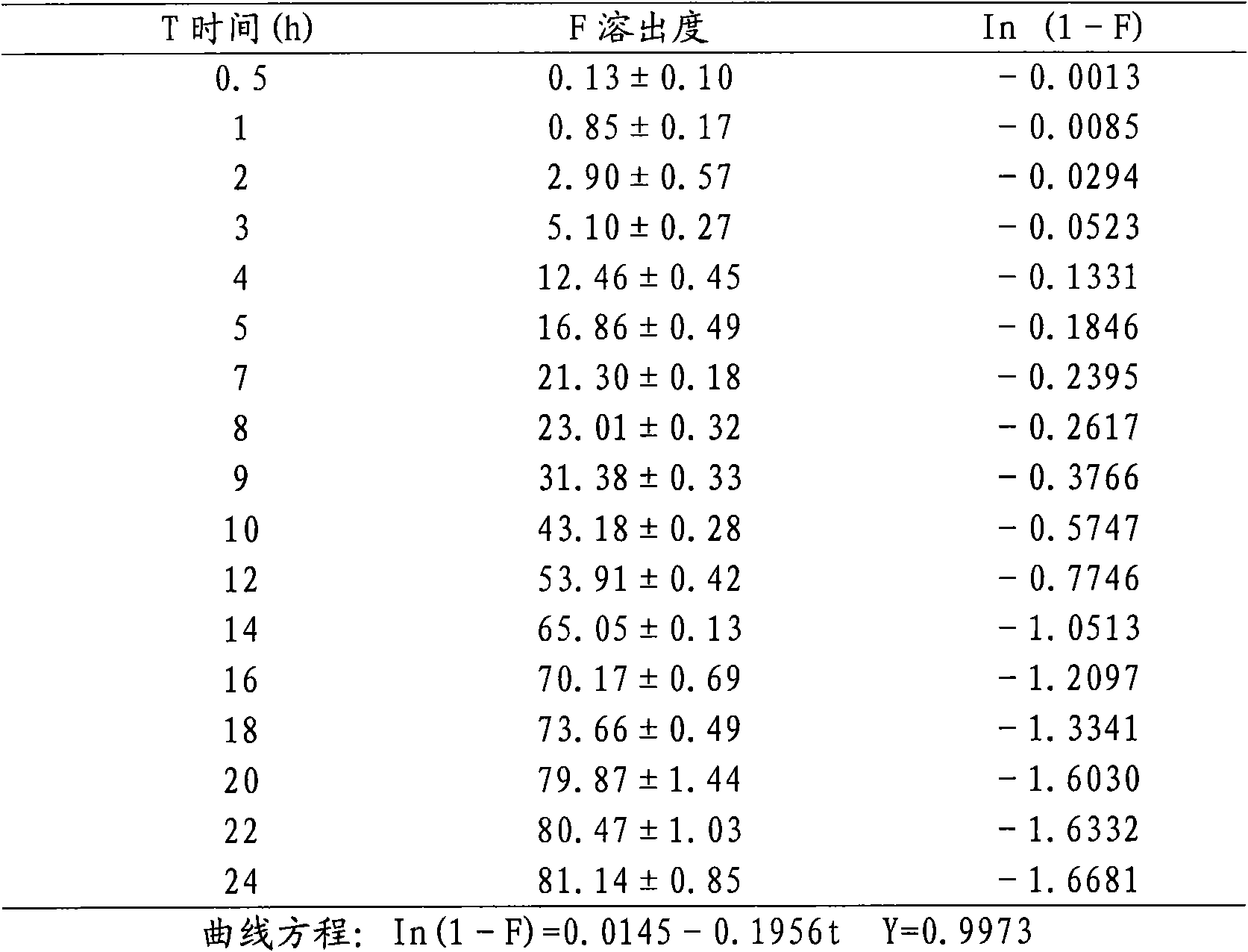
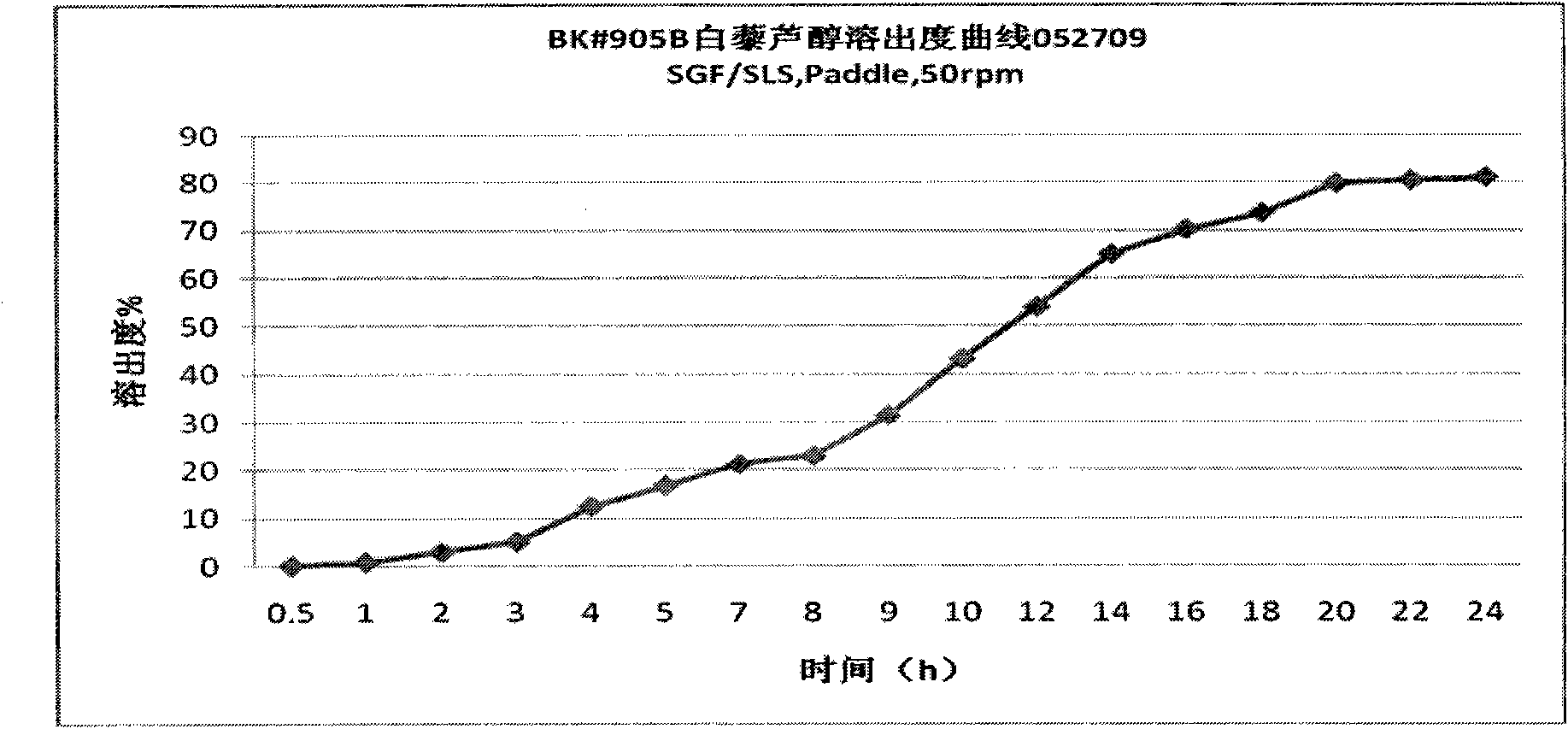
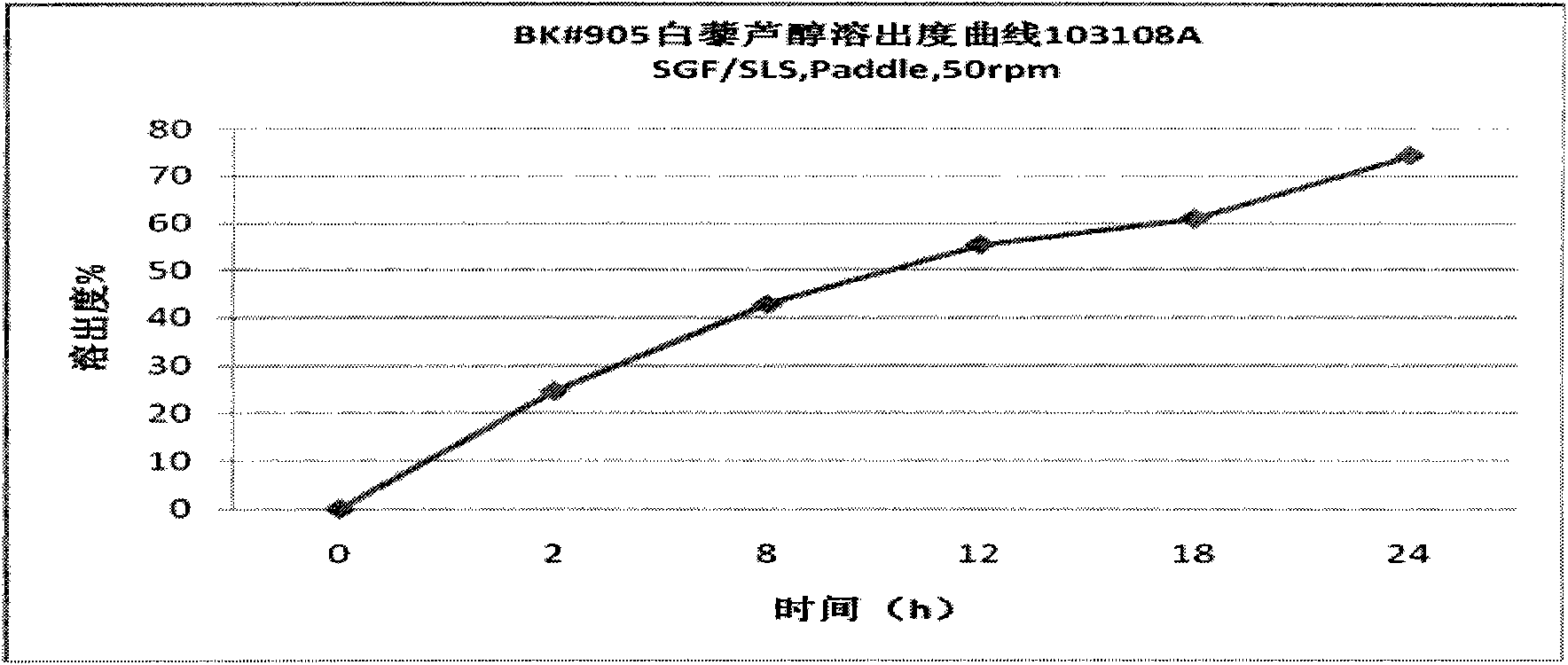
[0024] The active ingredients are resveratrol, quercetin, anthocyanins and proanthocyanidins, accounting for 73.75% by weight; microcrystalline cellulose in the carrier is 20%, ethyl cellulose is 5%, and polyvinylpyrrolidone is 1.5% , and the sum of the three is 26.5%; the aqueous solution with alcohol is mixed uniformly with a high-speed shear mixer, sieved or extruded; dried and made into granules.
Embodiment 2
[0026] Get active ingredient resveratrol, quercetin 52.5%; In the carrier, ethyl cellulose is 36.7%, polyacrylic acid resin 10.1%, the sum of the two is 46.8%; 0.7% polyethylene glycol (PEG400) is dissolved in iso Propanol is mixed evenly with a high-speed shear mixer, sieved or extruded; dried and made into granules.
Embodiment 3
[0028] Any one or more of the active ingredients resveratrol, quercetin, anthocyanins, proanthocyanidins, dextran, flavonoids, tea polyphenols, and vitamins is 40%; the microcrystalline fiber in the carrier The element is 15%, the ethyl cellulose (EC) is 35%, the aqueous dispersion of ethyl cellulose (trade name Surelease) is 10%, and the aqueous solution is mixed with a high-speed shear mixer, sieved or extruded into Molded; made into granules after drying.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com