Influenza compound multi-epitope DNA vaccine and application thereof
A DNA vaccine and multi-epitope technology, applied in the field of DNA vaccines, can solve the problems of unsatisfactory immune protection test results and large impact
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
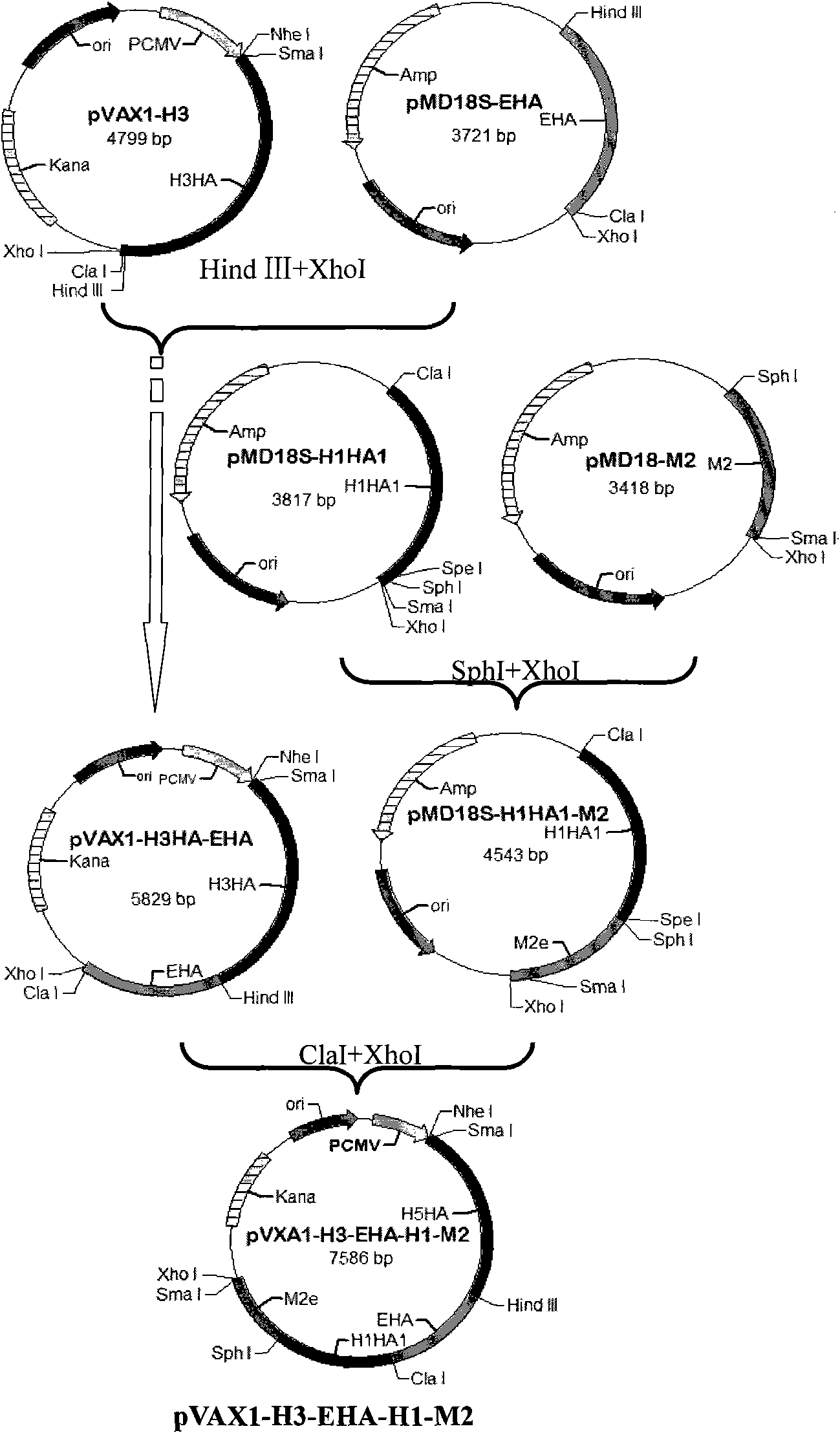
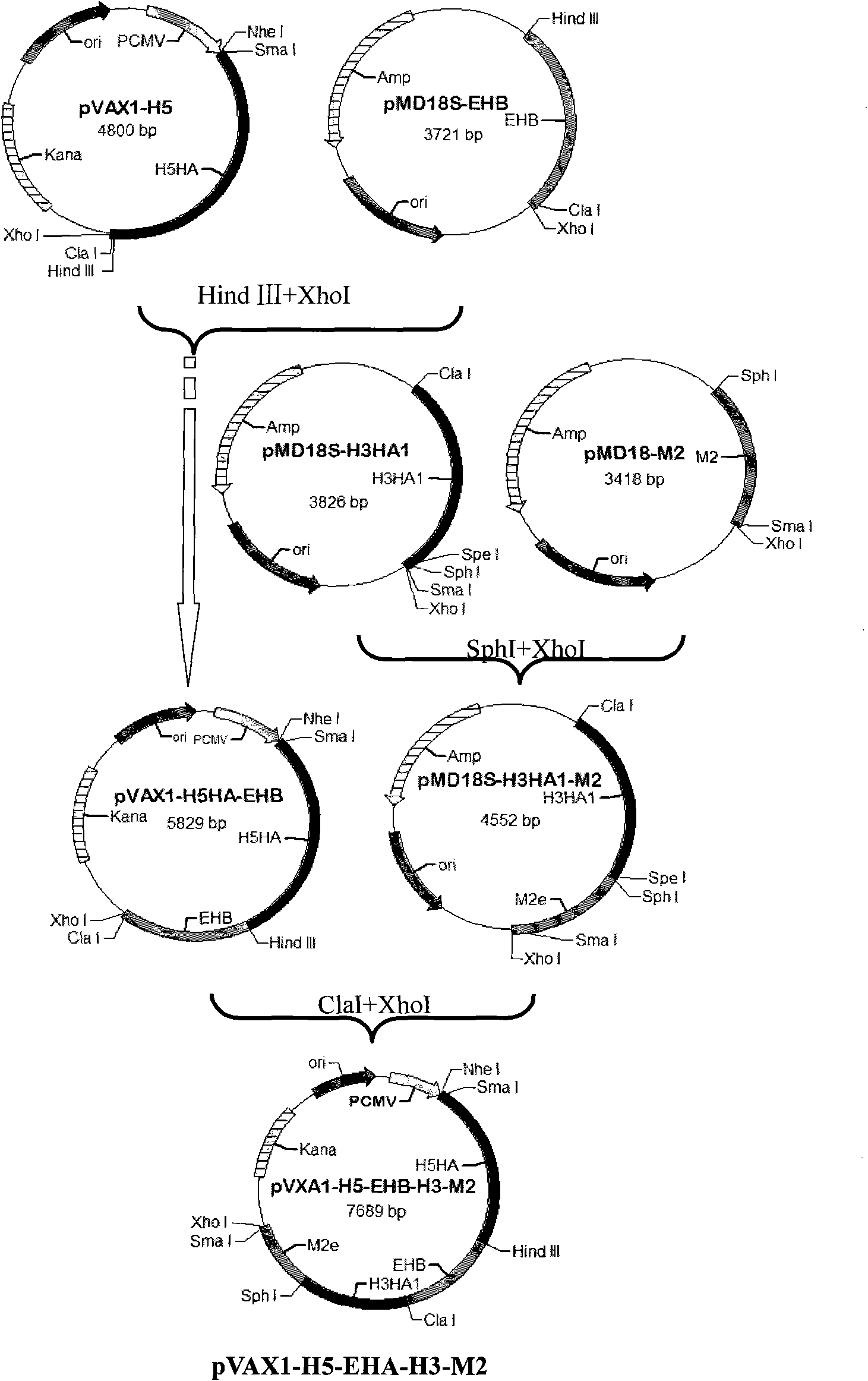
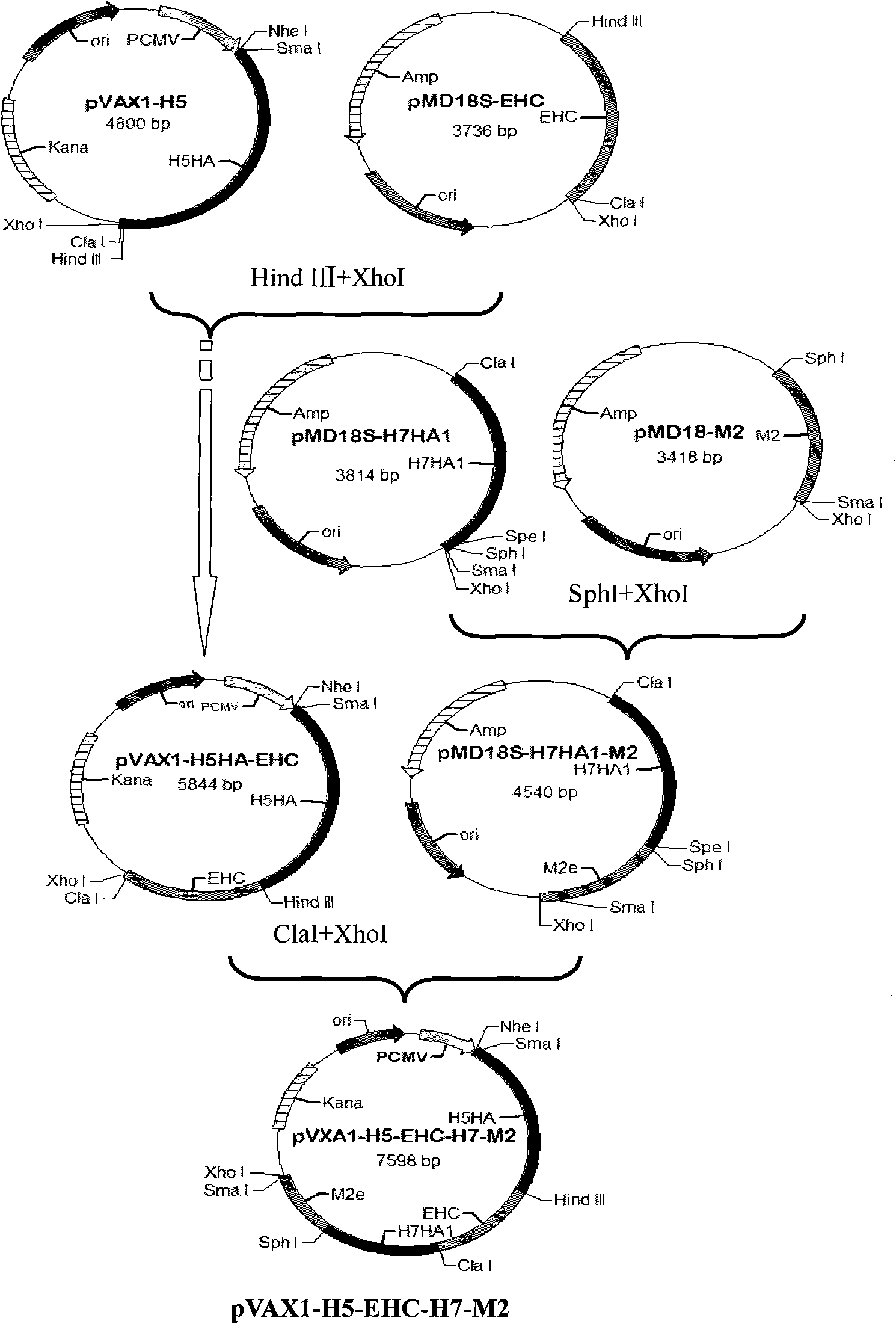
[0052] The establishment of embodiment 1 influenza multi-epitope DNA vaccine
[0053] Collect the published influenza epitopes, refer to the existing research before the present invention, and download the H1, H3, H5, H7, H9 subgroups respectively from NCBI (http: / / www.ncbi.nlm.nih.gov) Amino acid sequences of HA and NA genes of influenza type 1.
[0054]Bioinformatics MHCI molecular prediction uses: web server SYPEITHI (http: / / syfpeithi.bmi-heidelberg.com), Bimas (http: / / www-bimas.cit.nih.gov / molbio / hla_bind), Multipre (http : / / antigen.i2r.a-star.Edu.sg); bioinformatics MHC class II molecule prediction uses: web server SYFPEITHI and Multipre; bioinformatics B cell epitope prediction uses: web server Bcepred (http: / / www .intech.res.in / raghava / bcepred) and biomolecular simulation software Insight II (Accelrys, 2005); proteasome cleavage prediction using: web server PAProC (http: / / www.paproc.de).
[0055] Using bioinformatics methods, the multi-subtype (H1, H3, H5, H7, H9) inf...
Embodiment 2
[0135] Example 2 Experimental Immunization Study of Mice with H3 / H1 Dominant Composite Multi-epitope Nucleic Acid Vaccine
[0136] 1. Experimental grouping, mouse immunization and sampling
[0137] Twenty BALB / c female mice were randomly divided into 2 groups, 10 mice in each group, respectively pVAX1 empty plasmid control group and pVAX-MEGNp24 plasmid immunization group. Immunization was carried out three times with an interval of 14 days. Each time, 100 μg rDNA (dissolved in 100 μL sterile saline) was injected into the bilateral tibialis anterior muscle of the mice. Blood was collected 2 weeks after the second immunization, placed overnight at 4°C, centrifuged at 5000rpm for 10min, serum was collected, and stored at -20°C for testing. On the 10th day after the third immunization, the eyeballs were picked to take blood, and the cervical spine was dislocated to kill. The serum was coagulated and separated for ELISA to detect cytokines; at the same time, the spleen was asept...
Embodiment 3
[0154] Example 3 H5 / H3 dominant compound multi-epitope nucleic acid vaccine pig experimental immunization research
[0155] 1. Experimental grouping, pig immunization and sampling
[0156] Thirty 50-day-old Landrace pigs were randomly divided into 6 groups with 5 pigs in each group. They were divided into 5 immunization groups and 1 empty plasmid control group, and the groups were marked with different ear numbers. They were immunized three times on 0d, 21d and 35d respectively. For the first immunization, 2 mg rDNA-chitosan nanoparticle suspension was intramuscularly injected into the neck of each pig. After each immunization, 1 mg rDNA was administered. Blood was collected from the hypogastric vein on 0d, 14d, 21d, 35d, and 45d, and centrifuged at 5000r / min for 10min to collect serum for ELISA antibody determination. Ten days after the third immunization, the anticoagulated blood was aseptically collected from the hypogastric vein for the separation of peripheral blood l...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com