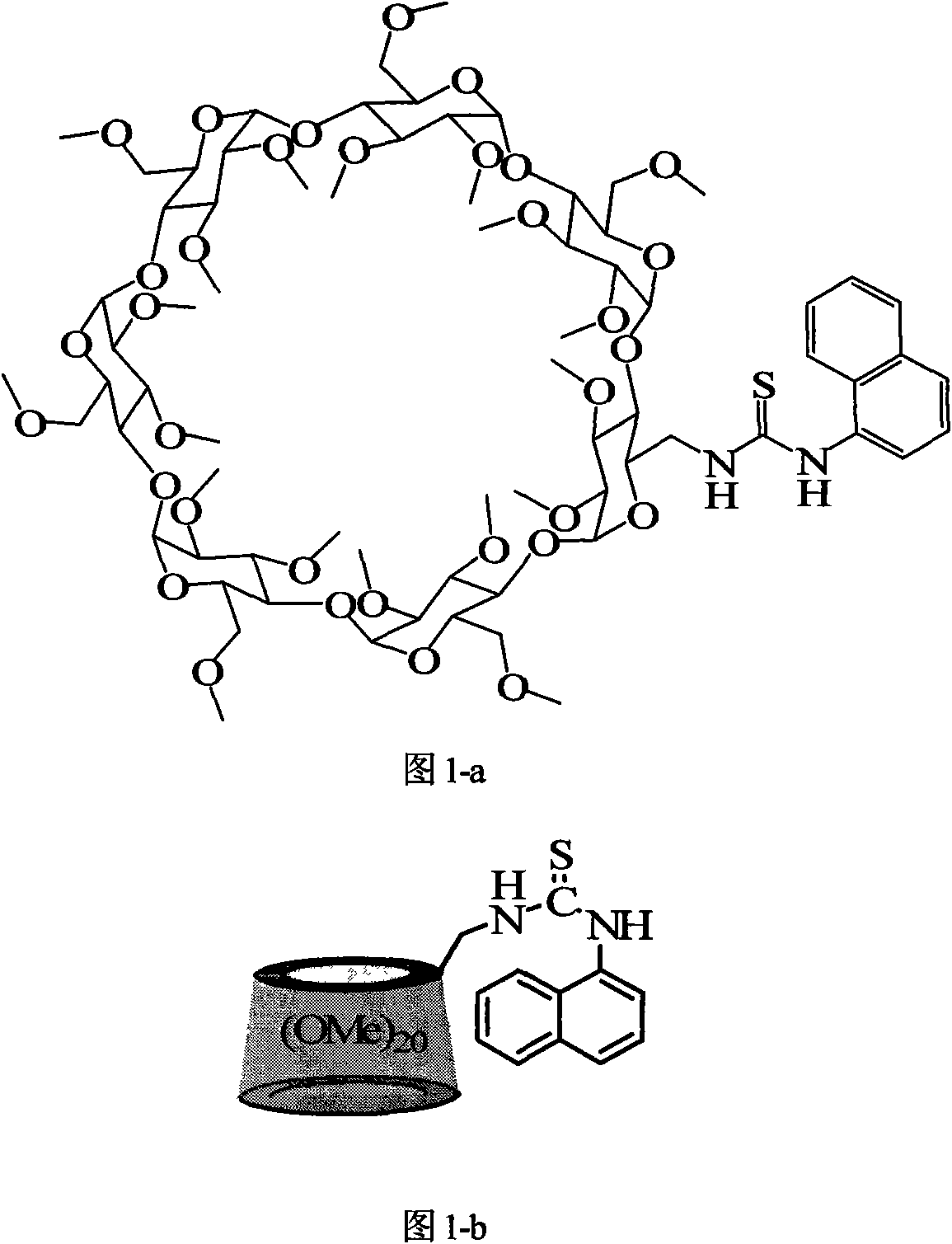
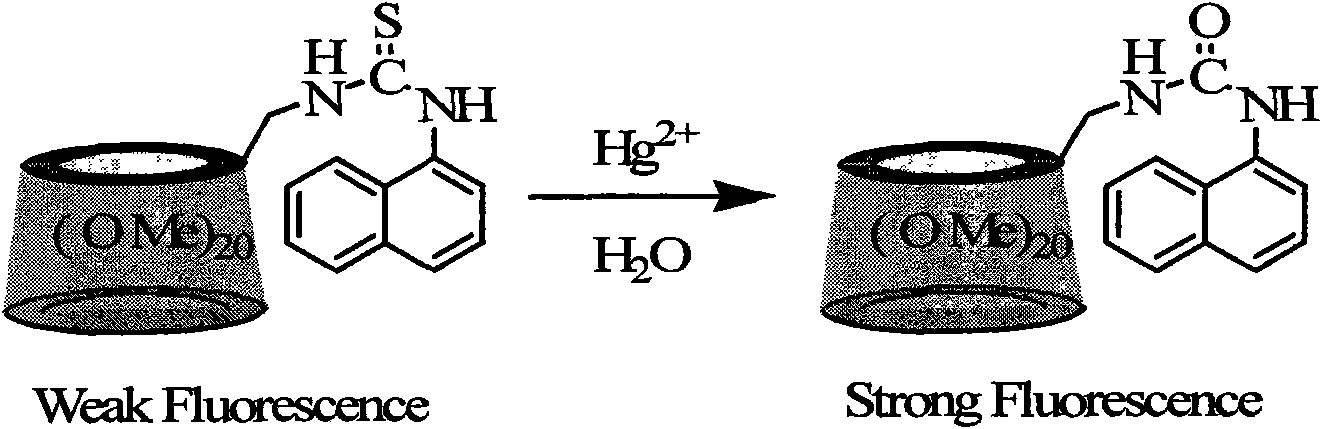
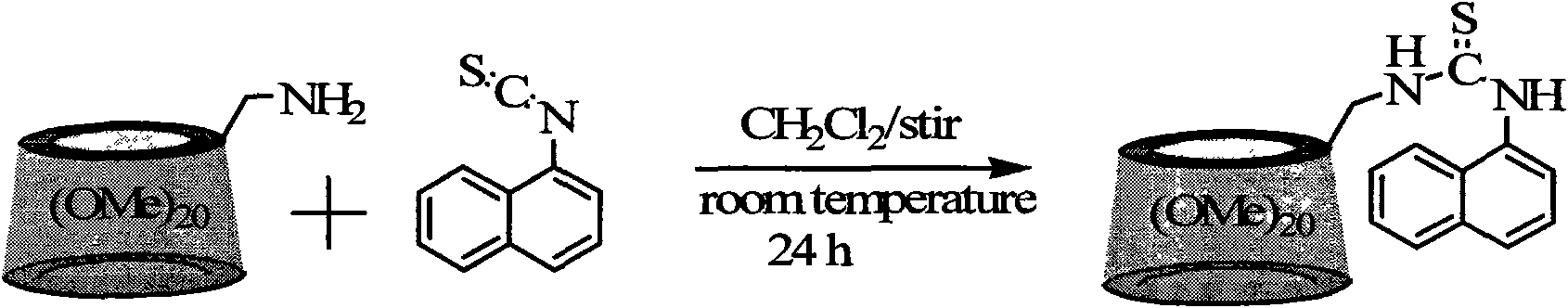Naphthylthiourea-modified permethylated beta-cyclodextrin derivative, preparation method and application method thereof
A technology of naphthyl thiourea and methylation, applied in fluorescence/phosphorescence, material excitation analysis, etc., can solve the problems of complex synthetic routes, low sensitivity, and long response time, and achieve simple synthetic routes, good sensitivity, and selectivity sex high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Synthesis of permethylated β-cyclodextrin derivatives modified by naphthylthiourea
[0037] Synthetic route such as figure 2
[0038] The synthesis steps are as follows:
[0039] Under a nitrogen atmosphere, 1.0 mmol 6-deoxy-6-amino-permethylated-β-cyclodextrin and 3.0 mmol 1-naphthyl isothiocyanate were reacted in a dry dichloromethane solution under electromagnetic stirring at room temperature After 24 hours, the solvent was removed by rotary evaporation. The obtained solid was separated with a silica gel column. The eluent was ethyl acetate to obtain a white naphthylthiourea modified permethylated β-cyclodextrin derivative with a yield of 85% and a molecular weight It is 1598.7g / mol.
Embodiment 2
[0041] Synthesis of permethylated β-cyclodextrin derivatives modified by naphthylthiourea
[0042] Synthetic route such as figure 2
[0043] The synthesis steps are as follows:
[0044] Under a nitrogen atmosphere, 1.0 mmol 6-deoxy-6-amino-permethylated-β-cyclodextrin and 30.0 mmol 1-naphthyl isothiocyanate were reacted in a dry dichloromethane solution under electromagnetic stirring at room temperature After 24 hours, the solvent was removed by rotary evaporation. The obtained solid was separated with a silica gel column. The eluent was ethyl acetate to obtain a white naphthylthiourea modified permethylated-β-cyclodextrin derivative with a yield of 86%. The molecular weight is 1598.7g / mol.
Embodiment 3
[0046] Compound characterization
[0047] The NMR, elemental analysis and mass spectrometric characterization of the naphthylthiourea modified permethylated β-cyclodextrin derivative of the present invention:
[0048] 1 HNMR(400MHz, D 2 O, ppm), δ3.06-3.66 (m, 101H, H of C-3, C-5, C-6, C-2, C-4, OC), 5.06-5.34 (m, 7H, H of C-1), 7.58-7.60 (m, 4H, H of naphthalene), 7.92-7.99 (m, 3H, H of naphthalene). C 73 H 118 N 2 O 34 S: Measured value: C, 54.50%; H, 7.76%; N, 1.72%. Theoretical value: C, 54.81%; H, 7.43%; N, 1.75%. ESI-MS: 1599.6[M+H] + , 1621.7[M+Na] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com