Bacterial strain and method for producing antibiotic avilamycin special for animal
A technology of avilamycin and antibiotics, which is applied in the field of strains for producing animal-specific antibiotic avilamycin, and can solve the problems of many key control points in the fermentation process, difficult to control the proportion of components, and low ability of bacteria to produce hormones. , to achieve the effect of ensuring safety, preventing animal diseases, and no cross-resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
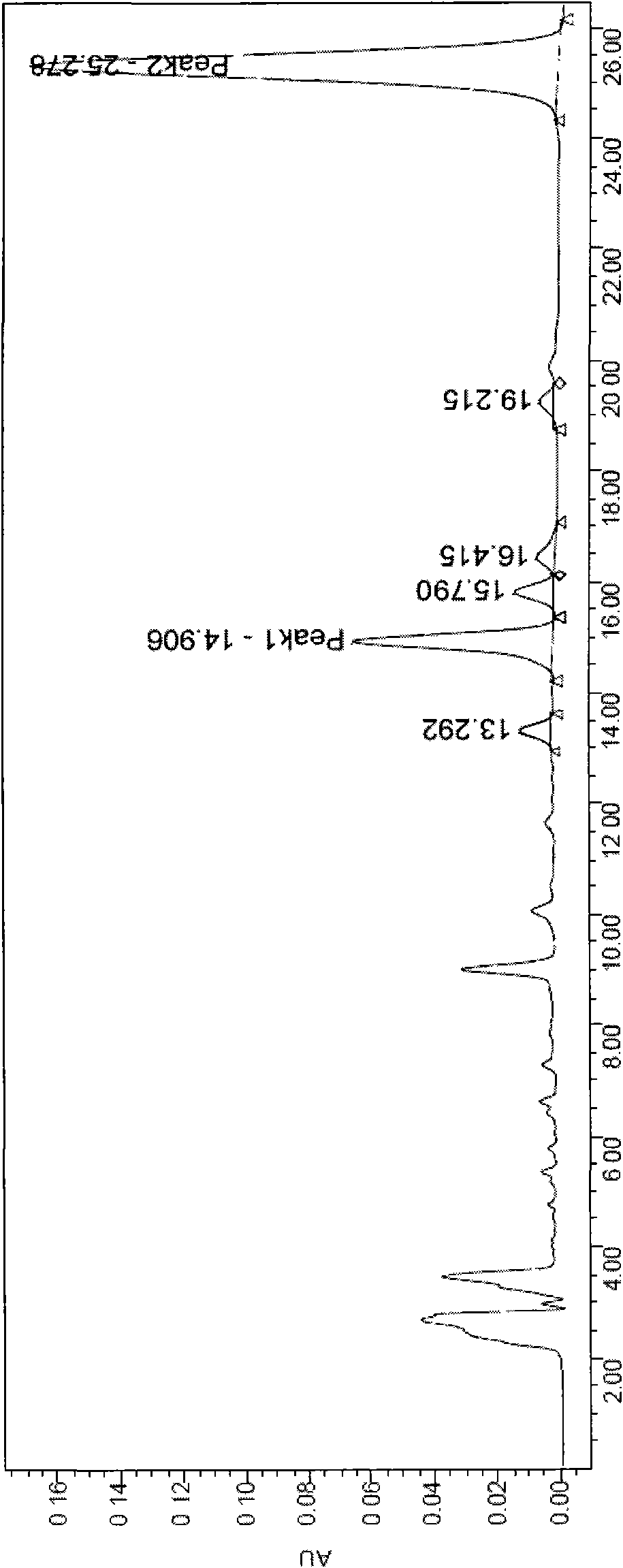
Image
Examples
Embodiment 1
[0042] The strain No. 104 adapted to the high-sugar medium was obtained by continuous ultraviolet mutagenesis of the wild strain SDSL1, and the operation steps were as follows:
[0043] Insert No. 1 strain into the slant medium, and after culturing for 8 days, select a well-grown test tube slant, add 10ml sterile water, wash 2 to 3 times, transfer to a small Erlenmeyer flask with glass beads to disperse and mix, Bacterial suspension, so that the cells are controlled at 10 8 A / ml or so. Put the suspension in a plate and expose it to a 30W ultraviolet light source at a distance of 15 cm for 60 seconds. Serial dilution 10 after irradiation -3 ~10 -5 , coated flat. The culture temperature was 28°C, and the culture time was 9 days. Colonies with good growth were selected for fermentation verification. At the same time, wild bacteria that have not been irradiated by ultraviolet rays are also subjected to fermentation verification. The fermentation medium was prepared with two...
Embodiment 2
[0051] Strain No. 104 was screened for streptomycin resistance to obtain strain No. 232. The 232 strain was mutated by nitrosoguanidine, and the high-yielding strain was screened in combination with streptomycin resistance. The operation steps are as follows:
[0052] Insert the No. 232 strain into the slant medium, and after culturing for 8 days, select a well-grown test tube slant, add 10ml of sterile water, wash 2 to 3 times, transfer to a small Erlenmeyer flask with glass beads to disperse and mix, Bacterial suspension, so that the cells are controlled at 10 8 A / ml or so.
[0053] In a fume hood, weigh 20 mg of nitrosoguanidine, add acetone at a ratio of 0.1 ml / mg to dissolve it, and then dilute it with 4 times citrate buffer, so that the concentration of the solution is 2 mg / ml. The spore suspension and the nitrosoguanidine solution were mixed at a ratio of 1:2, and acted at 26-28°C for 30 minutes. After the treatment, centrifuge, wash and centrifuge more than 10 time...
Embodiment 3
[0062] The No. 1009 strain that had been screened by mutagenesis was purified naturally through several rounds. The spore suspension used is prepared with example 1, and concentration is about 10 8 pieces / ml. The operation is as follows:
[0063] Select 8 thick test tubes, add 9 ml of sterile water to No. 1-8, draw 1 ml of spore suspension into No. 1 tube, shake and mix well, suck 1 ml from No. 1 tube to No. 2 tube, and so on, to make Gradient concentration. Draw 0.05ml from No. 6 tube and smear evenly in the plate culture medium. In the same way, draw 0.1 and 0.2ml of the dilution solution from tubes 7 and 8, respectively, and put them in the plate culture medium. The culture temperature was 28°C, and the culture time was 8 days. Select a single colony that is robust and free of bacteria and insert it into the slant culture, pass the seed culture, and then carry out fermentation verification.
[0064] (1) Plate medium: cornstarch 25g / L, KNO 3 1g / L, K 2 HPO 4 0.5g / L, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com