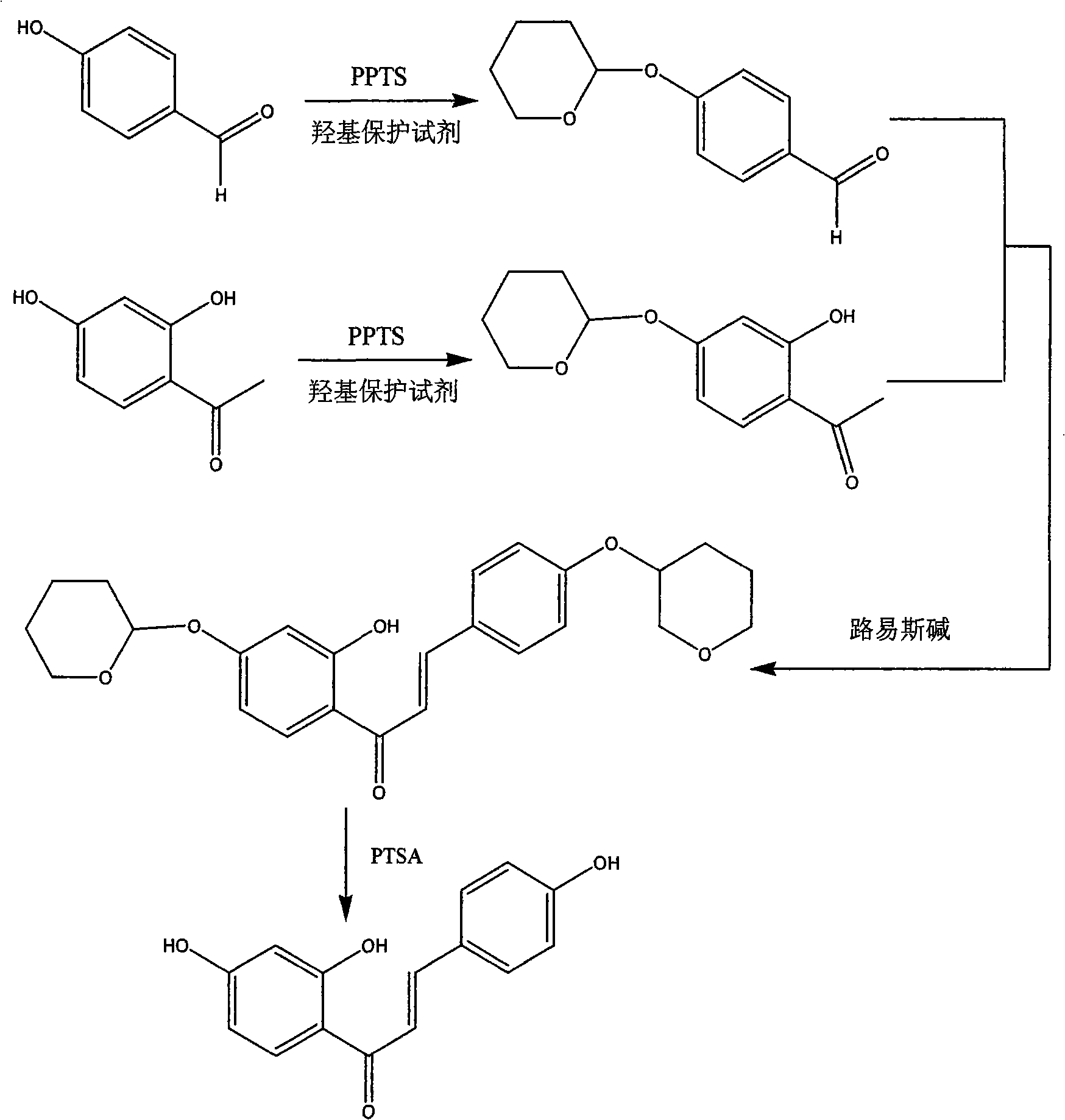
Method for synthesizing isoliquiritigenin
A technology of isoliquiritigenin and aldol condensation, which is applied in the fields of chemical instruments and methods, preparation of organic compounds, carbon-based compounds, etc., to achieve the effect of simple method, mild reaction conditions, and easy-to-obtain raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] (1) Hydroxyl protection of 4-hydroxybenzaldehyde: add 3.63g of 4-hydroxybenzaldehyde and 0.52g of pyridinium p-toluenesulfonate into a round bottom flask, add dichloromethane and stir well, slowly add 4.2ml of 3,4-bis Hydropyran, then stirred at 30°C, detected by TLC. After 5 hours, the reaction was completed. The solvent in the system was evaporated under reduced pressure, the residue was diluted, washed with saturated brine, and the organic phase was dried, and the solvent was evaporated under reduced pressure to obtain 5.27 g of an oily product with a yield of 86%.
[0026] (2) 4-hydroxyl protection of 2,4-dihydroxyacetophenone: 6.39g2,4-dihydroxyacetophenone and 0.36g pyridinium p-toluenesulfonate were added to the round bottom flask, and dichloromethane was added to stir Uniformly, slowly add 5.70ml of 3,4-dihydropyran, then stir at 30°C for 4h, add 2.4g of sodium bicarbonate, continue to stir for 4h, filter off the sodium bicarbonate, and concentrate the filtrate ...
Embodiment 2
[0034] (1) Hydroxyl protection of 4-hydroxybenzaldehyde: 3.89g 4-hydroxybenzaldehyde and 0.56g pyridinium p-toluenesulfonate were added to a round bottom flask, chloroform was added and stirred evenly, and 3.62ml chloromethylformazol was slowly added ether, then stirred at 35°C and detected by TLC. After 4 hours, the reaction was completed. The solvent in the system was evaporated under reduced pressure, the residue was diluted, washed with saturated brine, and the organic phase was dried, and the solvent was evaporated under reduced pressure to obtain 5.26 g of an oily product with a yield of 80%.
[0035] (2) 4-hydroxyl protection of 2,4-dihydroxyacetophenone: 4.14g2,4-dihydroxyacetophenone and 0.23g pyridinium p-toluenesulfonate were added to the round bottom flask, and chloroform was added to stir Evenly, slowly add 3.07ml of chloromethyl methyl ether, then stir at 35°C for 3h, add 1.55g of sodium bicarbonate, continue to stir for 4h, filter out the sodium bicarbonate, and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com