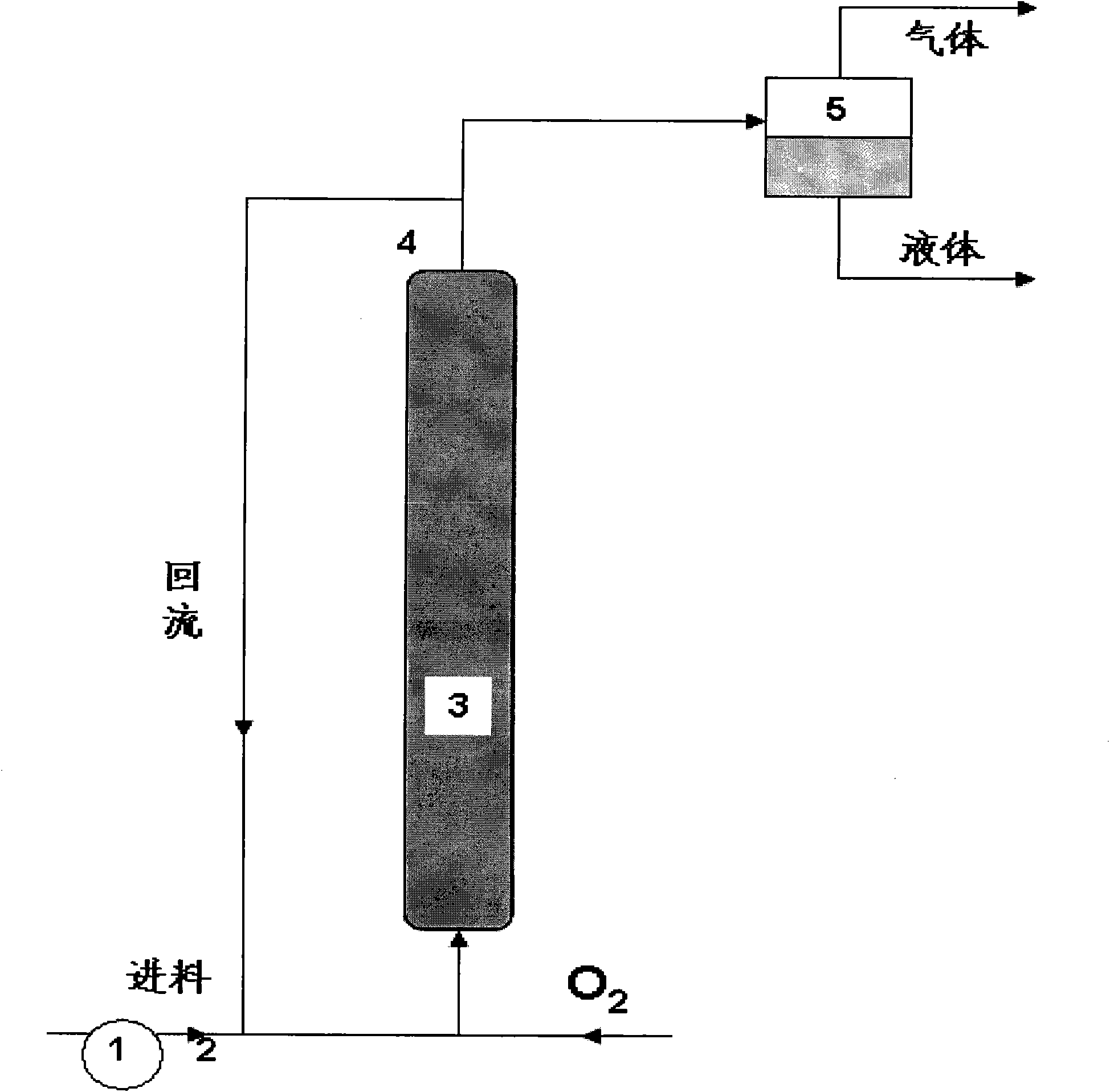
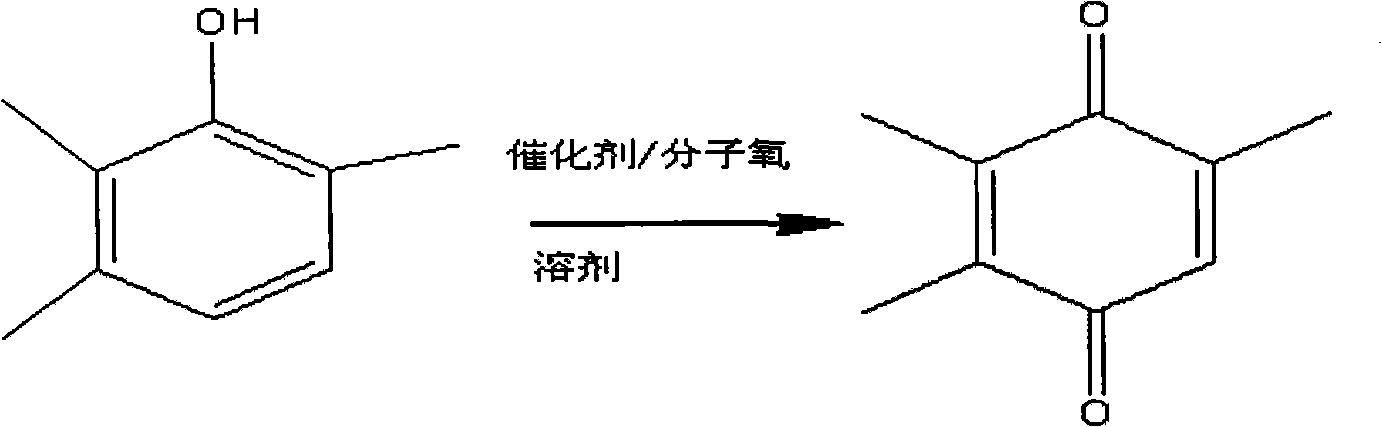
Method for preparing trimethylbenzoquinone with resin-supported catalyst
A technology of trimethylbenzoquinone and trimethylphenol, applied in the field of producing trimethylbenzoquinone, can solve the problems of long reaction time, large energy consumption, unfavorable industrialization, etc., and achieve good mechanical strength, good thermal stability, The effect that is not easy to lose
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Add 2.0g of macroporous anion exchange resin (D301) supported catalyst ([Cu 2+ ] / [N] molar ratio = 0.5), 20.0 g n-butanol and 1.36 g trimethylphenol (0.01 mol), add a certain oxygen pressure (1 bar), and stir vigorously (1000 rpm) at the same time. The reaction was maintained at 100°C for 8 hours. After the reaction, the molar yield of trimethylbenzoquinone was determined to be 94.5% by liquid chromatography.
Embodiment 2
[0032] After the completion of Example 1, the stirring was stopped, and the solvent was directly poured out by the decant method. The residual solids were washed three times with the solvent to become the recovered catalyst.
[0033] Add the recovered catalyst, 20.0 g n-butanol and 1.36 g TMP (0.01 mol) into a 100 ml four-neck flask, add a certain oxygen pressure (1 bar), and stir vigorously (1000 rpm) at the same time. The temperature was maintained at 60° C. and the reaction was conducted for 6 hours. After the reaction, the molar yield of trimethyl benzoquinone was 92.4% as determined by liquid chromatography.
Embodiment 3
[0035] In a 100ml four-necked flask was added 2.0g of gel anion exchange resin (201) supported catalyst ([Cu 2+ ] / [N] molar ratio = 0.25), 20.0 g ethylene glycol butyl ether and 1.36 g TMP (0.01 mol), add a certain oxygen pressure (1 bar), and stir vigorously (1000 rpm) at the same time. Maintain the temperature at 80° C. and react for 21 hours. After the completion of the reaction, the molar yield of trimethyl benzoquinone was calibrated by liquid chromatography to be 64.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com