Hydrogel controlled by double switch of hydrogen bond and π-π stacking and its preparation and application
A hydrogel, π stacking technology, applied in the field of drug controlled release system research, can solve the problem of inability to match drug metabolism
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
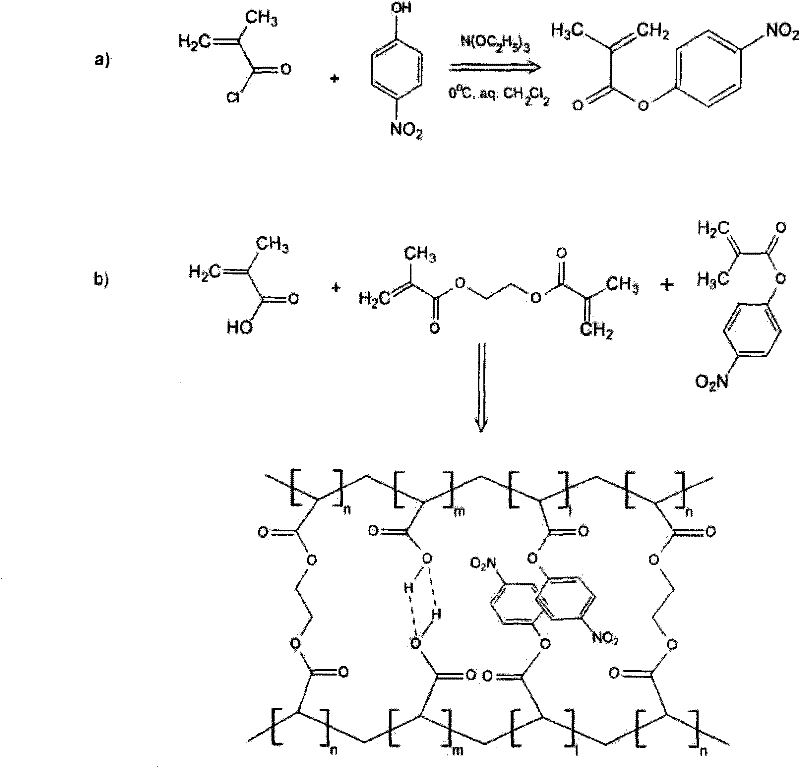
[0023] 1. Preparation of functional monomers
[0024] (1) p-nitrophenol methacrylate
[0025] Dissolve methacryloyl chloride, p-nitrophenol and triethanolamine (1:1:1 molar ratio) in chloroform, stir and react for 5-60 hours at a low temperature of -10-5°C (such as an ice-water bath), and wash with saturated NH 4 Cl ends the reaction, and the reaction product is recrystallized from ethanol to obtain white crystals. The resulting product is dried below 100°C for future use.
[0026] (2) p-nitrophenol acrylate
[0027] Dissolve acryloyl chloride, p-nitrophenol and triethanolamine (1:1:1 molar ratio) in chloroform, stir and react at a low temperature of -10 to 5°C (such as an ice-water bath) for 5 to 60 hours, and wash with saturated NH 4 Cl ends the reaction, and the reaction product is recrystallized from ethanol to obtain white crystals. The resulting product is dried below 100°C for future use.
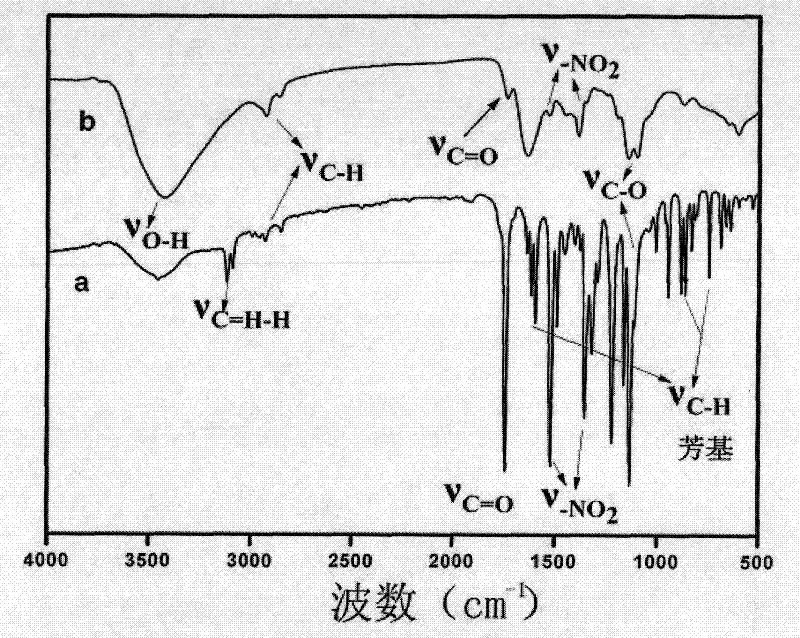
[0028] The proton magnetic resonance spectrum of the product that the synth...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com