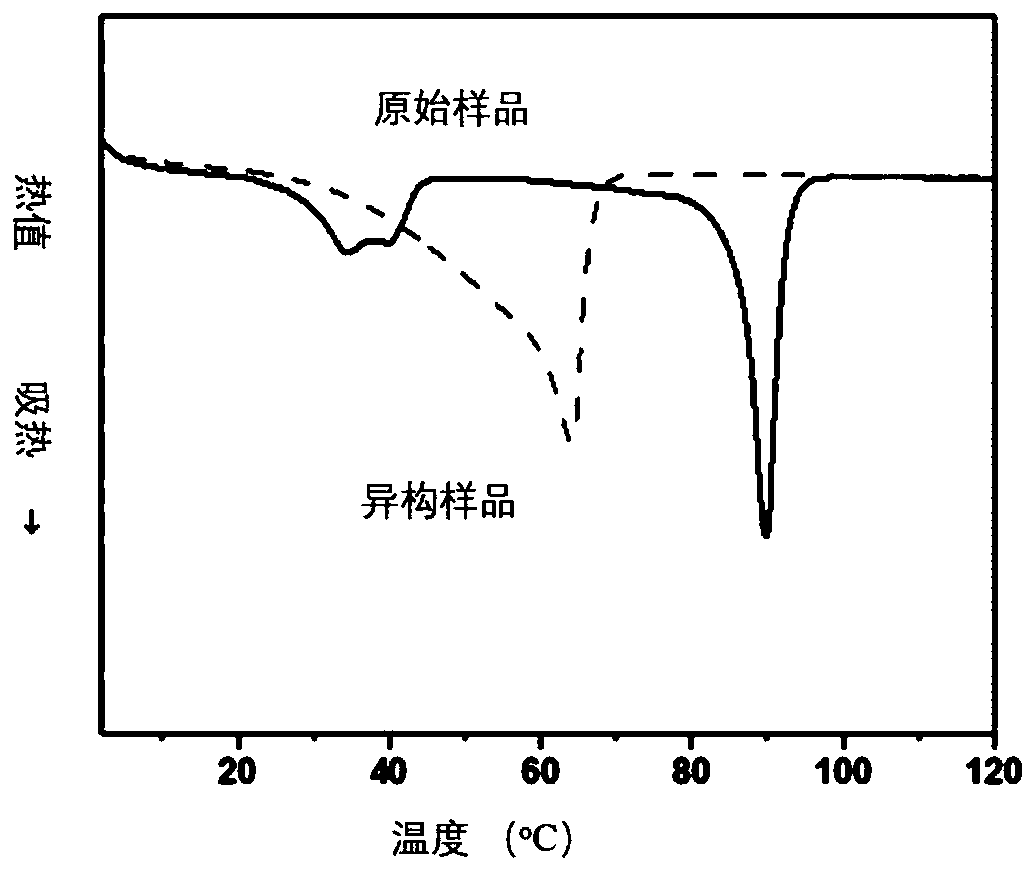
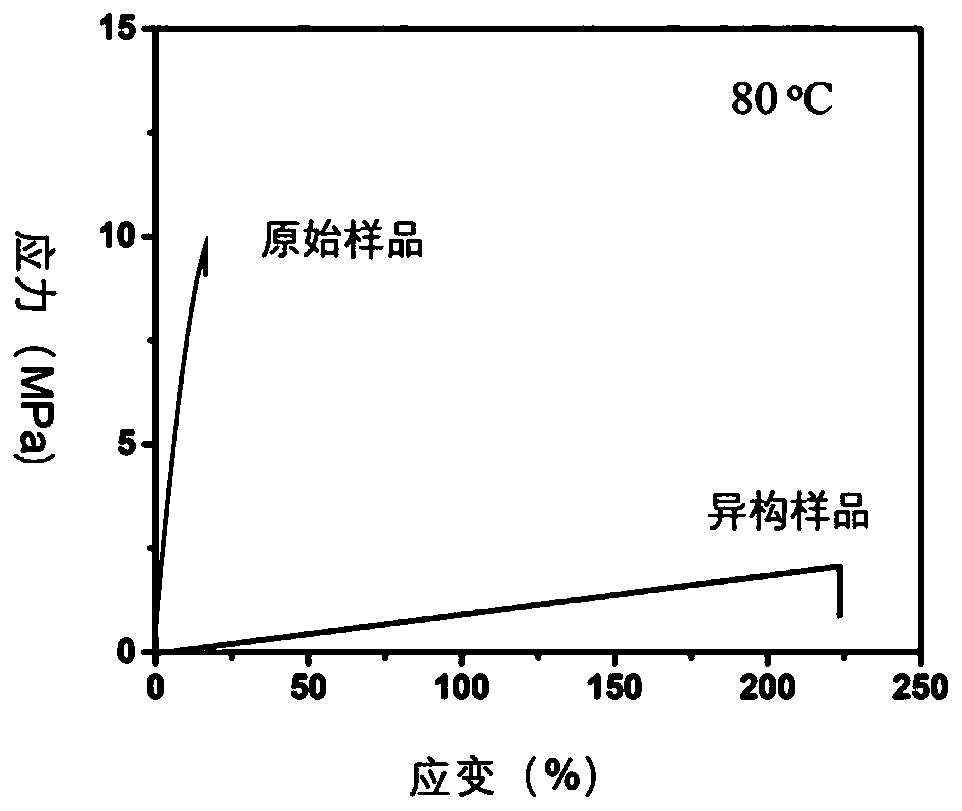
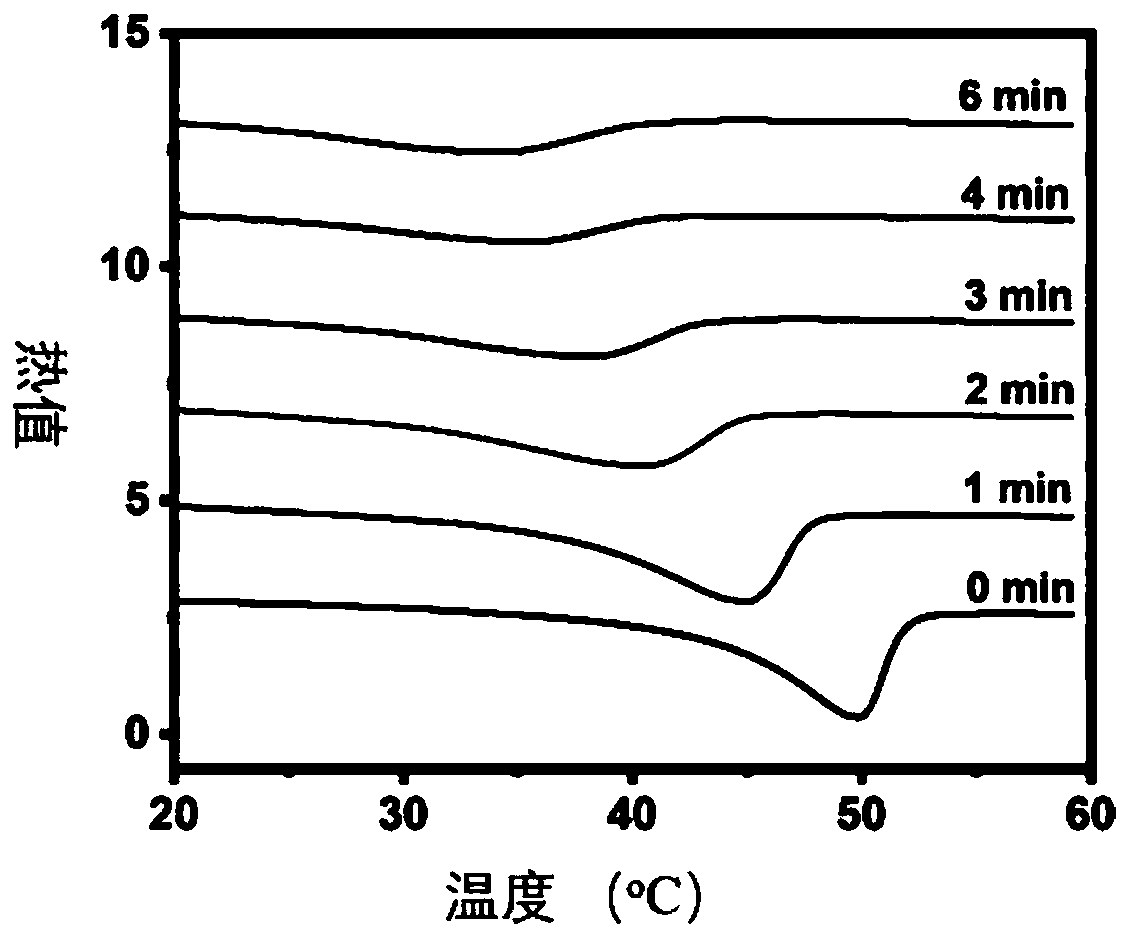
Polymer network topology heterogeneous system based on dynamic covalent bonds and application method thereof
A technology of dynamic covalent bonding and network topology, which is applied in the field of polymer network topological heterogeneous systems, can solve problems such as difficult to realize the change and regulation of polymer-related physical properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] raw material:
[0067] a) polycaprolactone diol (PCLDA): Mw=10000, Sigma-Aldrich company; Structural formula is as follows:
[0068]
[0069] b) polypentadecanolactone diol (PPDLDA): Mw=2000, Sigma-Aldrich company;
[0070] The structural formula is as follows:
[0071]
[0072] c) Hexamethylene diisocyanate (HDI): Sigma-Aldrich company; Structural formula is as follows:
[0073]
[0074] d) Dibutyltin dilaurate (DBTDL): TCI Company;
[0075] e) 1,5,7-Triazabicyclo[4.4.0]dec-5-ene: TCI Company;
[0076] f) Dichloroethane: Aladdin Reagent (Shanghai) Co., Ltd.;
[0077] Preparation:
[0078] Weigh 0.1mmol of polypentadecanolactone diacrylate, 0.9mmol of polycaprolactone diacrylate and 1mmol of hexamethylene diisocyanate in 10mL of dichloroethane (wherein PCL-diol and PPDL-diol The molar ratio of the total number of hydroxyl groups to the isocyanate group is 1:1), heated to 80C to dissolve. Then add dibutyltin dilaurate (its addition is 0.5% of the total ma...
Embodiment 2
[0081] raw material:
[0082] a) 2,2-bis[(2-propenyloxy)methyl]-1-butanol: TCI Company; the structural formula is as follows:
[0083]
[0084] b) caprolactone: TCI company; Structural formula is as follows:
[0085]
[0086] c) 3,6-dioxa-1,8-octanedithiol, TCI company; its structural formula is as follows:
[0087]
[0088] d) tetraallyloxyethane, TCI company; Its structural formula is:
[0089]
[0090] e) 1-hydroxycyclohexyl phenyl ketone (UV-184): TCI company;
[0091] f) stannous octoate: TCI company;
[0092] g) 1,5,7-Triazabicyclo[4.4.0]dec-5-ene / ketoprofen complex: TCI Company;
[0093] h) Toluene: Aladdin (Shanghai) Reagent Company;
[0094] i) n-Hexane: Aladdin (Shanghai) Reagent Company;
[0095] Preparation:
[0096] Weigh 0.35mol of caprolactone, 8mmol of 2,2-bis[(2-propenyloxy)methyl]-1-butanol and 0.5mmol of stannous octoate, and heat to 120°C for 10 hours under argon atmosphere . After the resulting product was dissolved in 50mL of toluene,...
Embodiment 3
[0099] raw material:
[0100] a) polyethylene glycol acrylate (PEGDA): molecular weight M n =3458, Sigma-Aldrich company; Structural formula enters as follows:
[0101]
[0102] b) hydroxyethylacrylamide: Sigma-Aldrich company; structural formula is as follows:
[0103]
[0104] c) benzophenone peroxide: Sigma-Aldrich company;
[0105] d) 1,5,7-Triazabicyclo[4.4.0]dec-5-ene: TCI Company;
[0106] e) N, N-dimethylformamide (DMF): TCI company;
[0107] Preparation:
[0108] Weigh 0.5g PEGDA and 0.07g hydroxyethylacrylamide in 0.5mL DMF to form a clear solution at 60°C, then add BPO (the amount added is 1.5% of the system mass) and 1,5,7-triazol Heterobicyclo[4.4.0]dec-5-ene / ketoprofen complex (the addition amount is 1.5% of the system mass). Then the precursor solution was transferred to a sealed glass tank and kept at 100°C for 24 hours. The resulting film was dried in a vacuum oven at 80 °C for 24 h.
[0109] Polymer network topology isomerization process: the sp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com