Method and device for burning chemistry chains based on iron or iron oxide
A technology of chemical chain combustion and iron oxide, which is applied in the direction of using non-combustion exothermic chemical reaction to generate heat, heating device, exothermic chemical reaction heat generation, etc., can solve the problems of wasting fuel gas and reducing purity, and achieve reduction Energy waste, improved purity, and easy capture effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
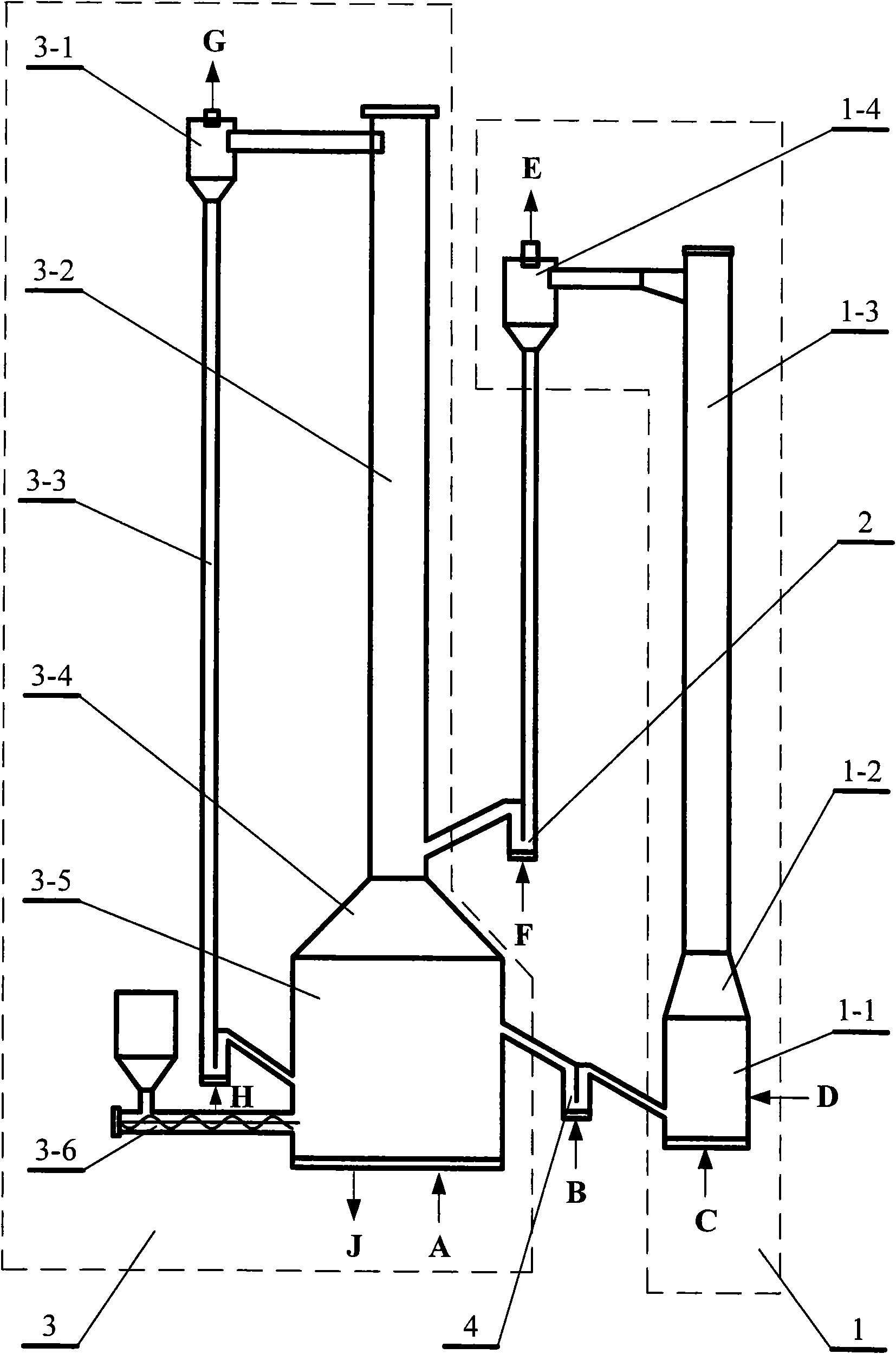
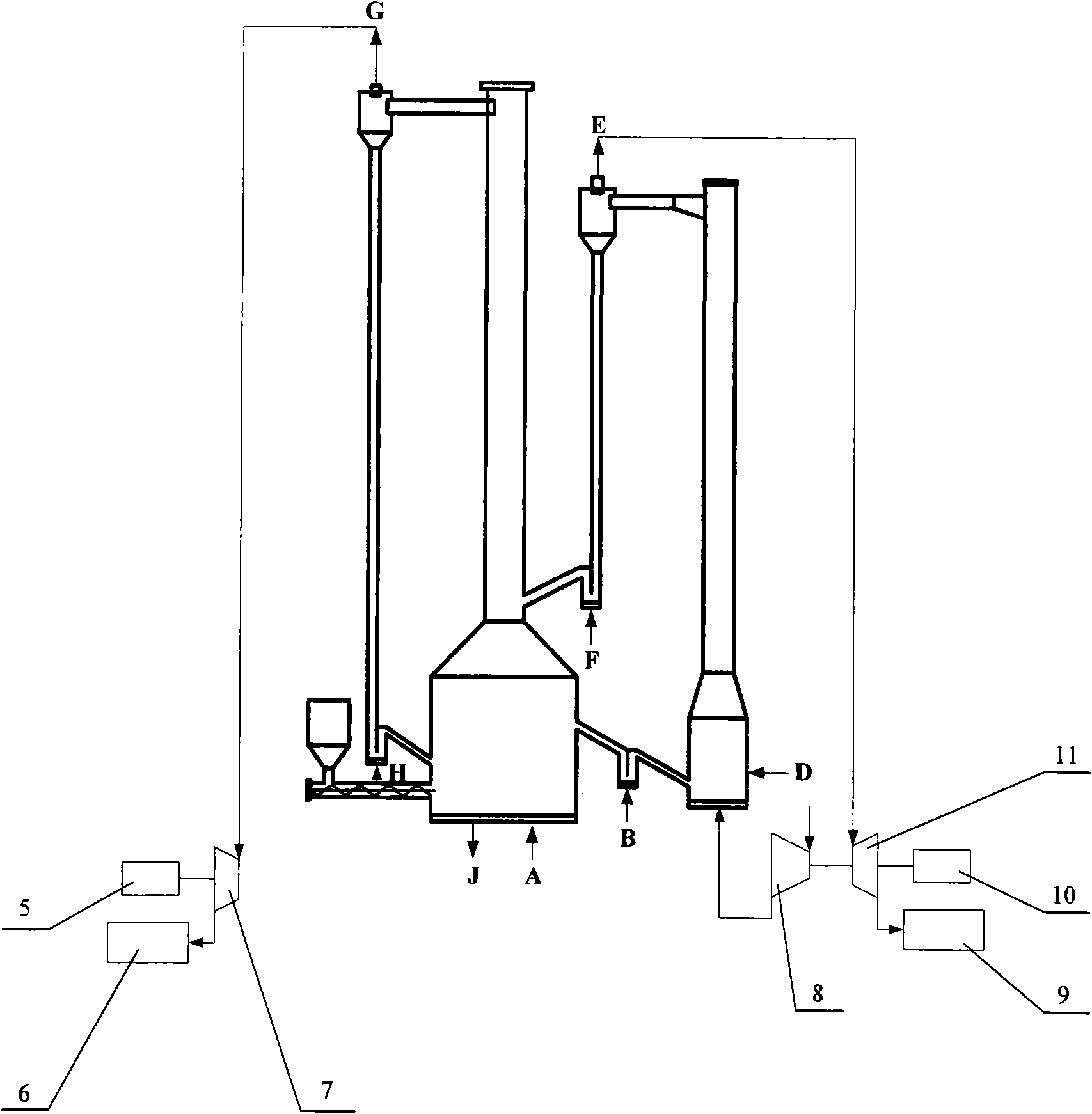
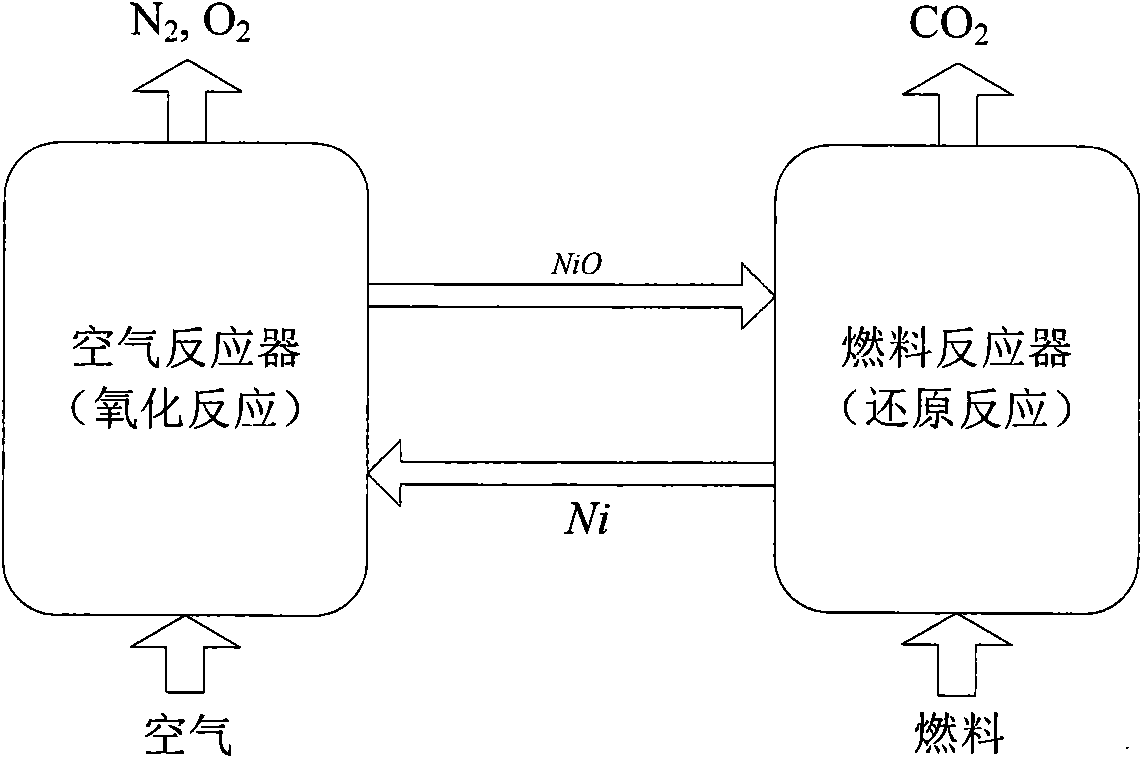
[0043] A method of chemical chain combustion based on iron or iron oxides using coal as fuel, placing iron or iron oxides in the fluidized bed 1 of the air reactor, and feeding fluidized air at the lower end C of the fluidized bed 1 of the air reactor , the operating temperature of the air reactor fluidized bed 1 can be controlled at about 800 ° C ~ 1250 ° C, iron or iron oxides react with oxygen in the air to obtain Fe 2 o 3 , and then the gas-solid two-phase is separated by the air separator 1-4; the separated high-temperature oxygen-poor air is discharged from the upper end E of the air separator 1-4, and is used for power generation or waste heat utilization; the separated Fe 2 o 3 Enter the fuel reactor fluidized bed 3 through the first overflow tank 2; the operating temperature of the fuel reactor fluidized bed 3 can be controlled at about 800 ° C ~ 1200 ° C; enter the fuel reactor riser 3 from the first overflow tank 2 Fe at the bottom of -2 2 o 3 Utilize the entrai...
Embodiment 2
[0045] A method of chemical looping combustion based on iron or iron oxides using natural gas as fuel, placing iron or iron oxides in the fluidized bed 1 of the air reactor, and feeding fluidized air at the lower end C of the fluidized bed 1 of the air reactor , the operating temperature of the air reactor fluidized bed 1 can be controlled at about 800 ° C ~ 1250 ° C, iron or iron oxides react with oxygen in the air to obtain Fe 2 o 3 , and then the gas-solid two-phase is separated by the air separator 1-4; the separated high-temperature oxygen-poor air is discharged from the upper end E of the air separator 1-4, and is used for power generation or waste heat utilization; the separated Fe 2 o 3 Enter the fuel reactor fluidized bed 3 through the first overflow tank 2; the operating temperature of the fuel reactor fluidized bed 3 can be controlled at about 800 ° C ~ 1200 ° C; The lower end A of the natural gas is passed into the natural gas, and enters the Fe at the bottom of ...
Embodiment 3
[0047]A device for realizing the method for chemical looping combustion of iron or iron oxides described in claim 1, comprising an air reactor fluidized bed 1, a first overflow tank 2, a fuel reactor fluidized bed 3 and a second The overflow tank is composed of 4. Air reactor fluidized bed 1 is composed of main reaction chamber 1-1, air reactor transition section 1-2, air reactor riser 1-3 and oxygen-poor air separator 1-4, air reactor riser 1 The lower end of -3 is connected with the main reaction chamber 1-1 through the air reactor transition section 1-2, and the upper end of the air reactor riser 1-3 is connected with the oxygen-poor air separator 1-4; the fuel reactor fluidized bed 3 It is composed of carbon dioxide separator 3-1, fuel reactor riser 3-2, return tank 3-3, fuel reactor transition section 3-4, mixed gasification reaction chamber 3-5 and screw feeder 3-6 , the lower end of the fuel reactor riser 3-2 is connected to the mixed gasification reaction chamber 3-5 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com