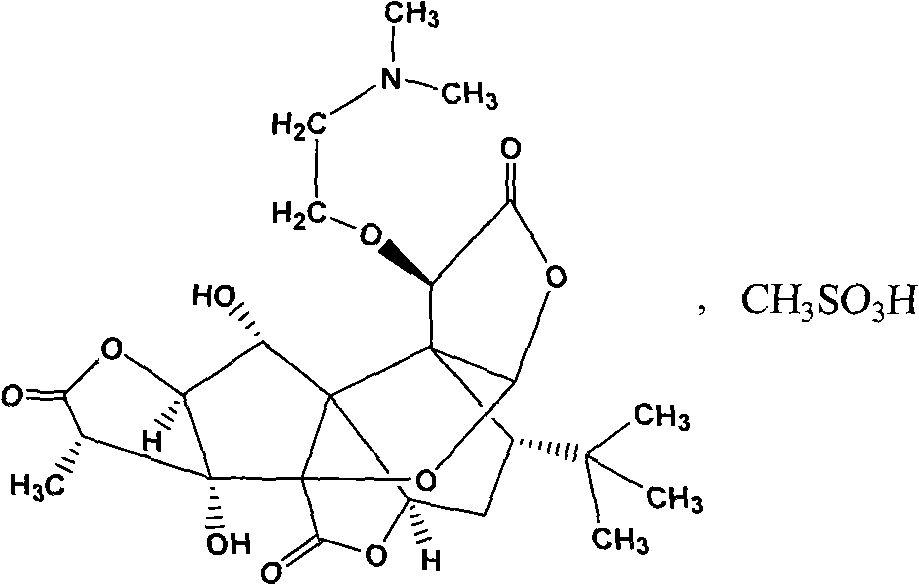
Method for analyzing high performance liquid chromatography of methanesulfonic amine ginkgolide B
A high-performance liquid chromatography, ginkgolide technology, applied in the direction of analysis of materials, material separation, measuring devices, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
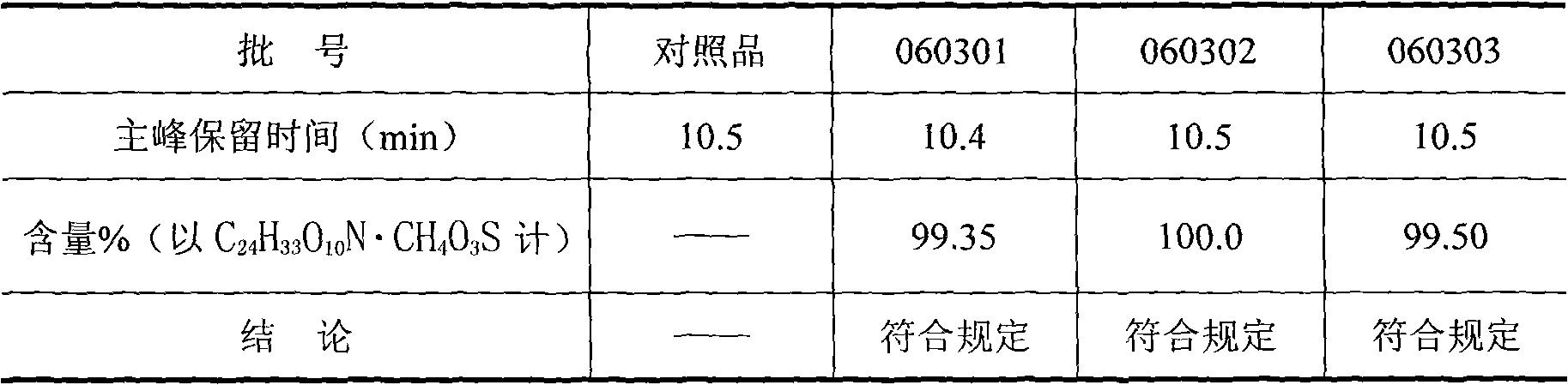
[0028] The identification and content determination of embodiment 1 mesylate amine ginkgolide B crude drug
[0029] Get need testing product and mesylate amine ginkgolide B reference substance appropriate amount, be mixed with water respectively the solution of 1.0mg / ml, for testing; To contain 5mmol / L sodium butanesulfonate and 20mmol / L sodium dihydrogen phosphate ( 1mol / L sodium hydroxide solution to adjust the pH to 7.8) aqueous solution-acetonitrile (volume ratio: 49 / 51) as the mobile phase; the chromatographic column is a C18 column; the column temperature is 25°C; the detection wavelength is 210nm; the flow rate is 1.0ml / min .
[0030] According to the external standard method, the content of the test product is calculated by the peak area of the main component; the identification is made according to whether the retention time of the main peak of the test product solution and the reference solution is consistent.
[0031] Identification and content determination resu...
Embodiment 2
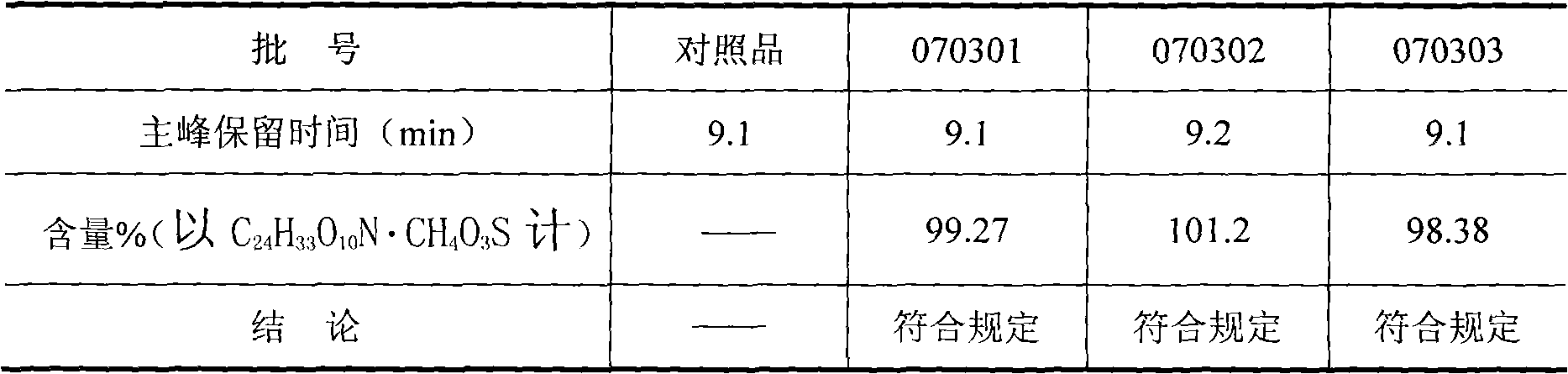
[0034] Identification and assay of embodiment 2 mesylate amine ginkgolide B sheet
[0035] Get an appropriate amount of ammonium ginkgolide mesylate B reference substance, dissolve with acetonitrile and be formulated with a solution of 1.0 mg / ml, as the reference substance solution; get an appropriate amount of test sample fine powder, dissolve the principal agent with acetonitrile and quantitatively dilute to formaldehyde-containing The solution of sulfonate amine ginkgolide B1.0mg / ml filters, and gets the continued filtrate as need testing solution; With the aqueous solution-acetonitrile (volume ratio: 45 / 55) as the mobile phase; the chromatographic column is a C18 column; the column temperature is 25°C; the detection wavelength is 205nm; the flow rate is 0.8ml / min.
[0036] According to the external standard method, the content of the test product is calculated by the peak area of the main component; the identification is made according to whether the retention time of th...
Embodiment 3
[0040] The identification and assay of embodiment 3 amine ginkgolide mesylate B for injection
[0041] Take 10mmol / L disodium hydrogen phosphate solution-methanol (volume ratio: 40 / 60) as the mobile phase; take an appropriate amount of ammonium ginkgolide mesylate B reference substance, dissolve it with the mobile phase and prepare a 1.0mg / ml solution, As reference substance solution; get need testing substance appropriate amount, dissolve and quantitatively dilute into the solution containing mesylate amine ginkgolide B1.0mg / ml with mobile phase, as need testing solution; Chromatographic column is C8 column; Column temperature 30 °C; detection wavelength is 215nm; flow rate is 1.2ml / min.
[0042] According to the external standard method, the content of the test product is calculated by the peak area of the main component; the identification is made according to whether the retention time of the main peak of the test product solution and the reference solution is consistent...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com