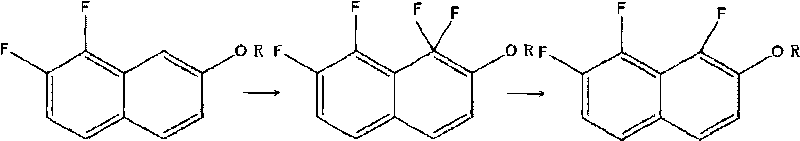
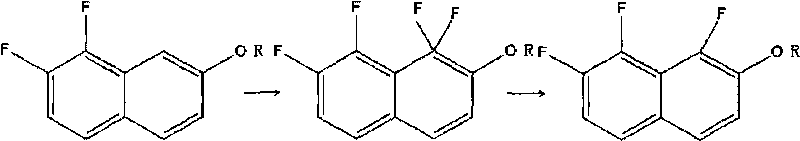
Preparation method of 1, 7, 8- trifluoro-2- naphthol and derivative thereof
A derivative, naphthol technology, applied in 1 field, can solve the problems of expensive palladium carbon catalyst, hydrogen hazard, etc., and achieve the effect of low cost, low price and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Embodiment 1: the preparation of 1,7,8-trifluoro-2-naphthol
[0020] 0.92g (0.005mol) of 7,8-difluoro-2-naphthol was dissolved in 35mL of acetonitrile in a 100mL three-necked flask, and heated to 50°C. Under continuous stirring, 5.35 g (0.0136 mol) of F-TEDA-BF4 was added, and the stirring was continued for 15 min. The solvent was rotovapped to obtain 0.54 g of a yellow solid, namely: 1,1,7,8-tetrafluoro-1,2-dihydronaphthalen-2-one.
[0021] Take 0.5 g of yellow solid, add 30 mL of acetonitrile, and heat to 50°C. Add 5mL of 0.95g (0.006mol) sodium formaldehyde sulfoxylate dihydrate (Sodium formaldehydesulfoxylate dihydrate) aqueous solution, and continue stirring at this temperature for 2.5h.
[0022] After filtering, the filtrate was separated into layers, and the organic phase was washed with 10 mL of 10% hydrochloric acid and washed with saturated brine three times, 5 mL each time. Dry over anhydrous sodium sulfate for 2 h, evaporate the solvent, and dry to obtain...
Embodiment 2
[0025] Embodiment 2: 1,7, the preparation of 8-trifluoro-2-naphthol
[0026] Take 0.92g (0.005mol) of 7,8-difluoro-2-naphthol and 35mL of acetonitrile into a 100mL three-necked flask, and raise the temperature to 50°C. Under constant stirring, 9.8 g (0.025 mol) of F-TEDA-BF4 was added. Stirring was continued for 20 min; a solid precipitated out. The solvent was rotovaped to obtain 0.48 g of a yellow solid. Namely: 1,1,7,8-tetrafluoro-1,2-dihydronaphthalene-2-one.
[0027] Take 1.0 g of 1,1,7,8-tetrafluoro-1,2-dihydronaphthalene-2-one, add 50 mL of acetonitrile, and heat to 55°C. Add 20mL of 3.8g (0.024mol) sodium formaldehyde sulfoxylate dihydrate aqueous solution. Stirring was continued at this temperature for 3h, resulting in a small amount of white solid.
[0028] After filtration, the filtrate was separated into layers, and the organic phase was washed with 30 mL of 10% hydrochloric acid, washed three times with saturated brine, dried over anhydrous sodium sulfate for ...
Embodiment 3
[0029] Embodiment 3: the preparation of 1,7,8-trifluoro-2-naphthol
[0030] Take 9.2g (0.05mol) of 7,8-difluoro-2-naphthol and 200mL of acetonitrile into a 500mL three-necked flask, and raise the temperature to 55°C. Under continuous stirring, 78.7 g (0.02 mol) of F-TEDA-BF4 was added. Stirring was continued for 30 minutes, and the reaction solution was filtered through celite.
[0031] The reaction solution obtained above in the fluorination step was heated to 50°C. Add 50mL of 7.3g (0.06mol) sodium formaldehyde sulfoxylate aqueous solution; continue stirring at this temperature for 2.5h, resulting in a small amount of white solid.
[0032] Filtration, the filtrate was separated, and the organic phase was taken, washed with 30 mL of 10% hydrochloric acid and then washed with saturated brine three times, dried over anhydrous sodium sulfate for 2 h, evaporated the solvent, and dried to obtain 7.3 g of a yellow solid. After recrystallization with toluene and petroleum ether [...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com