Thia-conjugated compound taking naphthalene tetracarboxylic acid diimide as kernel as well as preparation method and application thereof
A technology of naphthalene tetracarboxylic acid diimide and conjugated compounds, which is applied in the field of n-type organic semiconductor materials, can solve the problems of low electron mobility, difficulty in forming, and poor film-forming properties of OTFT devices, and achieve a simple and effective synthesis method , high purity, excellent performance and stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Example 1: N,N'-Dioctyl-[2,3-d:6,7-d']-bis[1,3-Dithia-2-yeryne-propanedicyano]-naphthalene - Synthesis of 1,4,5,8-tetracarboxylic diimide (1).
[0042] Concrete synthetic steps are:
[0043] 1.1 Synthesis of 2,3,6,7-tetrabromonaphthalene tetracarboxylic dianhydride (TBNDA)
[0044] 450mL 20% oleum was mixed into a 1L three-necked flask, and naphthalenetetracarboxylic dianhydride (NDA) (16g, 59.7mmol), liquid bromine (16mL, 320mmol) and 0.8g iodine (catalyst) were added thereto. Raise the temperature of the reaction mixture to 130°C, heat the reaction for 78 hours, then lower it to room temperature, remove unreacted liquid bromine with nitrogen flow, pour the reaction solution into 1Kg of crushed ice, filter, and wash the precipitate with a large amount of water until the filtrate is neutral. Drying in vacuo yielded 28 g of a yellow powdery solid (TBNDA), with a yield of 81%.
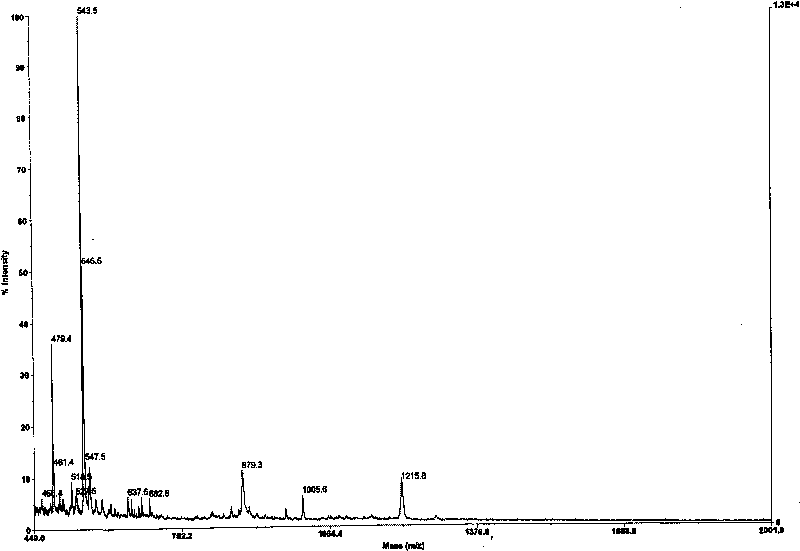
[0045] Mass Spectrum (MS-EI)) m / z 584 (M + , 95%)
[0046] 1.2 Synthesis of N,N'-dioctyl...
Embodiment 2
[0059] Example 2: N, N'-bis(2-ethyl-hexyl)-[2,3-d:6,7-d']-bis[1,3-dithia-2-yrylene- Synthesis of malondicyano]-naphthalene-1,4,5,8-tetracarboxylic diimide (2).
[0060] Concrete synthetic steps are:
[0061] 2.1 Synthesis of N, N'-bis(2-ethyl-hexyl)-2,3,6,7-tetrabromo-naphthalene tetracarboxylic acid diimide
[0062] Using 2-ethyl-hexylamine instead of n-octylamine, the synthesis method is the same as step 1.2 in Example 1, and the yield is 33%.
[0063] Mass Spectrum: [MS(TOF)]m / z: 806.8(M + ), 884.3(M+2K) + .
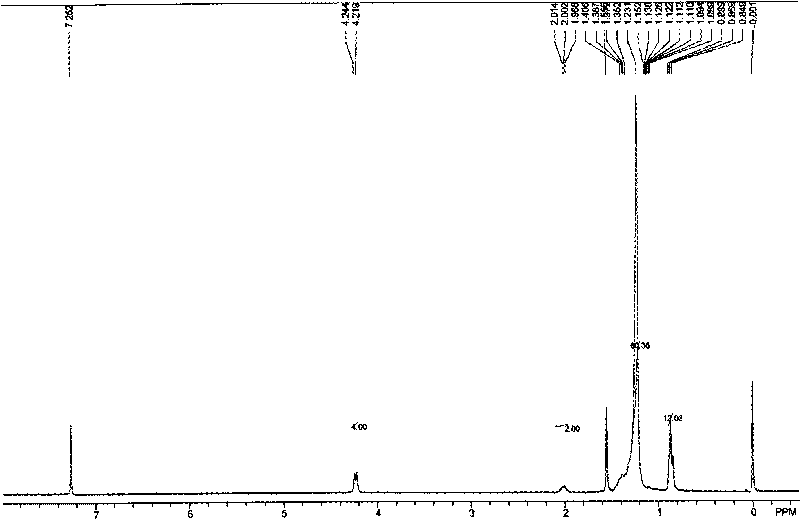
[0064] H NMR spectrum: 1 H-NMR (300MHz, CDCl 3 )δ (ppm): 0.859-0.969 (m, 6H), 1.287-1.409 (m, 8H), 1.923-1.967 (m, 1H), 4.169-4.194 (d, J=7.50Hz, 2H).
[0065] 2.2 Synthesis of example compound 2
[0066] Replace N, N'- Dioctyl-2,3,6,7-tetrabromonaphthalene-1,4,5,8-tetracarboxylic diimide, synthetic method is with step 1.4 in the embodiment 1, prepares dark brown solid (2 ), yield 51%.
[0067] Mass Spectrum: [MS(TOF)]m / z: 767.5(M + ).
[0068] Elemental ...
Embodiment 3
[0070] Example 3: N, N'-bis(2-octyl-dodecyl)-[2,3-d:6,7-d']-bis[1,3-dithia-2-phyllo Synthesis of orylene-propanedicyano]-naphthalene-1,4,5,8-tetracarboxylic diimide (3).
[0071] Concrete synthetic steps are:
[0072] 3.1. Synthesis of N,N'-bis(2-octyl-dodecyl)-2,3,6,7-tetrabromonaphthalene-1,4,5,8-tetracarboxylic diimide
[0073] Using 2-octyl-dodecylamine instead of n-octylamine, the synthesis method is the same as step 1.2 in Example 1, and the yield is 33%.
[0074] Mass spectrum: [MS(TOF)] m / z: 1144.8 (M + ).
[0075] H NMR spectrum: 1 H-NMR (300MHz, CDCl 3 )δ (ppm): 0.863-0.888 (m, 6H), 1.235 (b, 32H), 1.966-2.008 (m, 1H), 4.159-4.183 (d, J=7.20Hz, 2H).
[0076] 3.2 Synthesis of example compound 3
[0077] Replace N with N,N'-bis(2-octyl-dodecyl)-2,3,6,7-tetrabromonaphthalene-1,4,5,8-tetracarboxylic diimide, N'-dioctyl-2,3,6,7-tetrabromonaphthalene-1,4,5,8-tetracarboxylic diimide, the synthetic method is the same as step 1.4 in Example 1, and reddish brown is obt...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com