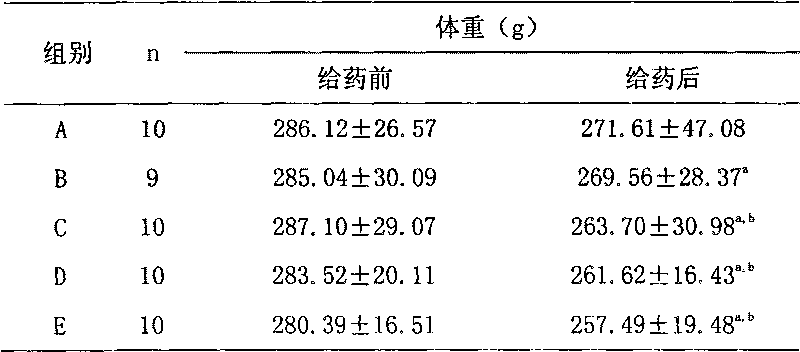
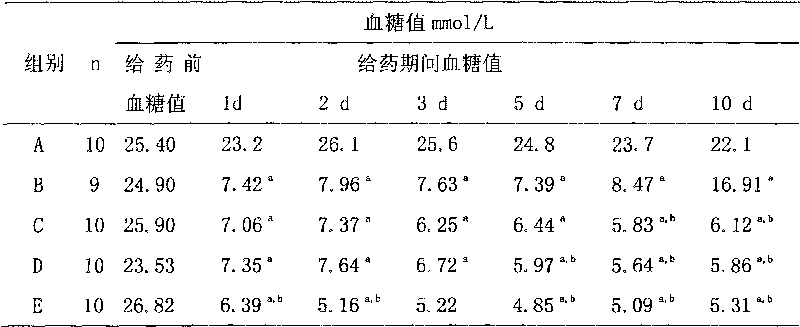
Medicine composite for treating B type diabetes
A technology of type 2 diabetes and composition, applied in the field of pharmaceutical composition for treating type 2 diabetes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Porcine insulin + cromolyn sodium oral microsphere preparation
[0020] Sodium cromoglycate 800mg is dissolved in 20ml of water for injection; Porcine insulin 6mg is dissolved in 30ml of 0.01mol / L hydrochloric acid; Sodium deoxycholate 4.0mg is mixed with disodium edetate 12.0mg; Acrylic resin II 600mg is dissolved in 20ml Soak in 90% ethanol overnight to dissolve. Mix the above four mixtures evenly, mix with 700ml liquid paraffin for 30min under stirring at 600r / min, slowly add 150ml of 0.5% gelatin solution and stir for 20min until the microspheres are solidified. The precipitate was collected by centrifugation, washed three times with an appropriate amount of petroleum ether, and dried under reduced pressure at 50°C for 12 hours to obtain porcine insulin cromolyn sodium enteric-coated microspheres.
[0021] The particle size distribution analysis is carried out with a scanning electron microscope image analyzer, and the average particle size is 4.96 μm, and the numb...
Embodiment 2
[0023] Recombinant human insulin + cromolyn sodium oral microsphere preparation
[0024] Sodium cromoglycate 600mg was dissolved in 10ml of water for injection; recombinant human insulin 3mg was dissolved in 20ml of 0.01mol / L hydrochloric acid; sodium deoxycholate 2.0mg was mixed with disodium edetate 6.0mg; acrylic resin II 300mg was dissolved in Soak in 20ml 90% ethanol overnight to dissolve. Mix the above four mixtures evenly, mix with 500ml liquid paraffin for 30min under stirring at 600r / min, slowly add 100ml of 0.5% gelatin solution and stir for 20min until the microspheres are solidified. The precipitate was collected by centrifugation, washed three times with an appropriate amount of petroleum ether, and dried under reduced pressure at 50°C for 12 hours to obtain recombinant human insulin cromolyn sodium enteric-coated microspheres.
[0025] The particle size distribution analysis is carried out with a scanning electron microscope image analyzer, and the average parti...
Embodiment 3
[0027] Porcine insulin + ketotifen fumarate oral microsphere preparation
[0028] 15 mg of ketotifen fumarate and 10 mg of porcine insulin were mixed evenly, and 4.0 g of sodium deoxycholate was added and mixed evenly; 300 g of chitosan (molecular weight was 3.84~6.12×10 5 ) was dissolved in 400ml of dichloromethane, then the above solid mixture was added into the solution, and ultrasonically emulsified for 30min. Add the emulsion to 850ml of 1.5% polyvinyl alcohol solution (W / V), stir at room temperature at 600r / min for 12h, and form microspheres. The particle size distribution analysis is carried out with a scanning electron microscope image analyzer, the average particle size is 5.39 μm, the number of microspheres in the range of 4-8 μm exceeds 62%, and the encapsulation efficiency is 75%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com