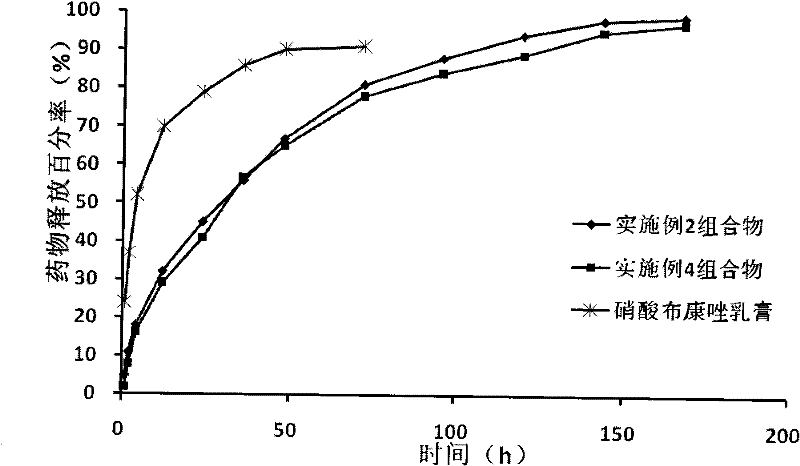
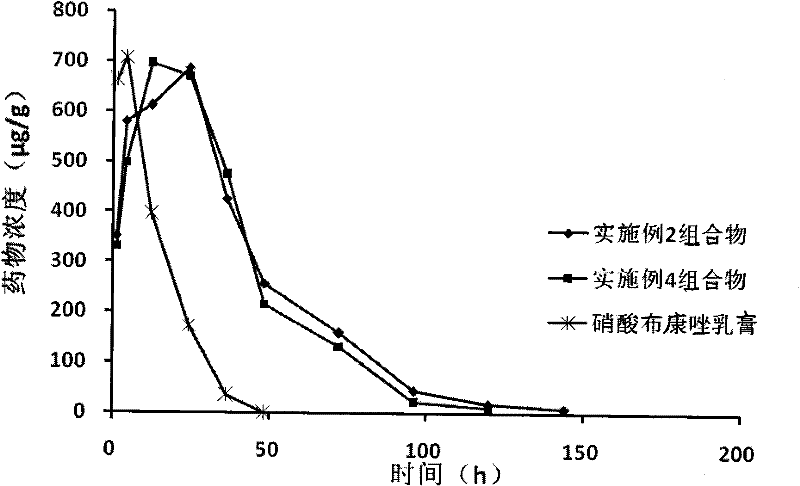
Medicinal composition for vagina
A composition and vaginal technology, which is applied in the directions of drug combinations, pharmaceutical formulas, and non-active ingredients medical preparations, etc., can solve the problems of reducing the patient's medication compliance, poor patient medication compliance, and short drug action time, and achieves the benefits of disease. Treatment, significant heat sensitivity, efficacy in improving medication compliance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] Embodiment 1. pharmaceutical composition components are as follows:
[0062] Butoconazole Nitrate 2%
[0063] Polyethylene glycol 16%
[0064] Glycerin 8%
[0065] Poloxamer 407 22%
[0066] Hypromellose K15M 1%
[0067] Carbomer 940 0.2%
[0068] Methylparaben 0.1%
[0069] Propylparaben 0.1%
[0070] water balance
[0071] Preparation:
[0072] 1. Take methylparaben and propylparaben, add water and continue to stir to dissolve, add carbomer while stirring, add hypromellose and poloxamer after dissolution, and place it at 4°C to make Sufficient swelling, as a gel matrix;
[0073] 2. Add butoconazole nitrate into polyethylene glycol and glycerin, stir for 2 hours, and use it as a liquid containing medicine;
[0074] 3. While stirring, add the drug-containing solution into the gel matrix, mix evenly, adjust the pH to 3-7 with 0.1mol / l NaOH solution and 0.1mol / l HCl solution if necessary, and pack it into the vaginal applicator , 5g each, that is.
Embodiment 2
[0075] Embodiment 2. The pharmaceutical composition components are as follows:
[0076] Butoconazole Nitrate 2%
[0077] Hydroxypropyl Beta Cyclodextrin 32%
[0078] Poloxamer 407 15.5%
[0079] Hypromellose K100M 0.6%
[0080] Carbomer 974 0.1%
[0081] Methylparaben 0.12%
[0082] Propylparaben 0.06%
[0083] water balance
[0084] Preparation:
[0085] Take carbomer, add water and continue stirring to dissolve, add butoconazole nitrate, hydroxypropyl beta cyclodextrin, methylparaben, propylparaben, add poloxamer, hypromellose while stirring Cellulose, placed at 4°C, after fully swollen, if necessary, use 0.1mol / l NaOH solution and 0.1mol / l HCl solution to adjust the pH to 4-6, and pack it in a vaginal applicator, 5g per Branch, that is.
Embodiment 3
[0086] Embodiment 3. pharmaceutical composition components are as follows:
[0087] Butoconazole Nitrate 2%
[0088] Hydroxypropyl Beta Cyclodextrin 25%
[0089] Poloxamer 407 18%
[0090] Hypromellose K100M 0.3%
[0091] Carbomer 980 0.1
[0092] Propylene Glycol 5%
[0093] Methylparaben 0.1%
[0094] Propylparaben 0.5%
[0095] water balance
[0096] Preparation:
[0097] Take butoconazole nitrate, hydroxypropyl beta cyclodextrin, methylparaben, propylparaben, add water and propylene glycol, mix well and continue stirring to dissolve, while stirring, add carbomer, poloxamer, Hypromellose, placed at 4°C, after fully swelling, if necessary, use 0.1mol / l NaOH solution and 0.1mol / l HCl solution to adjust the pH to 3-7, and pack it into the vaginal applicator , 5g each, that is.
PUM
| Property | Measurement | Unit |
|---|---|---|
| transition temperature | aaaaa | aaaaa |
| phase transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com