Method for preparing vapreotide
A technology of vapreotide and solid-phase synthesis method, which is applied to the preparation method of peptides, chemical instruments and methods, peptides, etc., can solve the problems of low application value, complicated operation, unfavorable industrial production, etc., and achieve low cost and high reaction rate. The effect of simple operation and less input of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
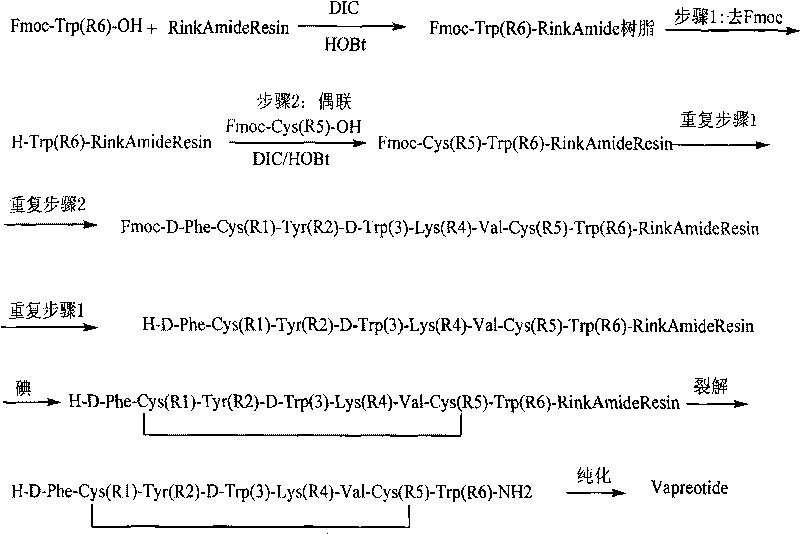
[0036] Embodiment 1, Fmoc-Trp (Boc)-RinkAmide resin preparation
[0037] 1. Put 50g Rink Amide Resin (Sub=0.8mmol / g) in a reaction bottle, add DMF to wash twice, and then swell with DMF for 30 minutes.
[0038] 2. Use 20% DBLK to remove the Fmoc protecting group of Rink Amide Resin, wash with DMF for 4 times and DCM for 2 times after removal, and detect with ninhydrin detection method, the resin is reddish brown.
[0039] 3. Weigh 63.2g of Fmoc-Trp(Boc)-OH and 19.5g of HOBT, add 500ml of DMF and 24.4ml of DIC, dissolve, add to the reaction bottle, and start the reaction.
[0040] 4. React for 2 hours, finish the reaction, wash with DMF three times, measure 56.4ml of acetic anhydride, 48.4ml of pyridine and mix them in 400ml of DMF, add to the reaction bottle to seal for two hours, then wash with DMF for 3 times, DCM for 2 times, methanol Washed twice, directly used for the next step of amino acid coupling.
Embodiment 2
[0041] Embodiment 2, the preparation of Fmoc-Cys(Trt)-Trp(Boc)-Rink Amide Resin
[0042] 1. After washing Fmoc-Trp(Boc)-Rink Amide Resin with DMF, remove Fmoc with 20% DBLK, then wash 4 times with DMF, wash 2 times with DCM, and detect with ninhydrin detection method. The resin is yellow.
[0043] 2. Weigh 70.2g Fmoc-Cys(Trt)-OH, 19.5g HOBT, add 500ml DMF and 24.4ml DIC, dissolve, add to the reaction bottle, and start the reaction.
[0044] 3. After 1.5 hours of reaction, the ninhydrin detection method was used to detect that the resin was transparent, and the reaction was terminated.
[0045] 4. After the reaction is completed, wash with DMF for 3 times, and couple amino acids one by one according to the peptide sequence of vapreotide until the last amino acid.
Embodiment 3
[0046] Embodiment 3, the oxidation of vapreotide
[0047] 1. Weigh 203.2g of iodine and dissolve it in 500ml of N,N-dimethylformamide. After the dissolution is complete, add it to the reaction bottle to start the oxidation reaction. The temperature of the reaction system is 35°C.
[0048] 2. Oxidation for 20 hours, the oxidation is over.
[0049]3. DMF was washed 5 times, DCM was washed 4 times, and finally methanol was added to wash 3 times, and dried to obtain 166 g of vapreotide-Rink Amide Resin.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com