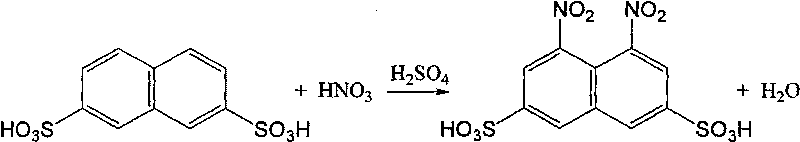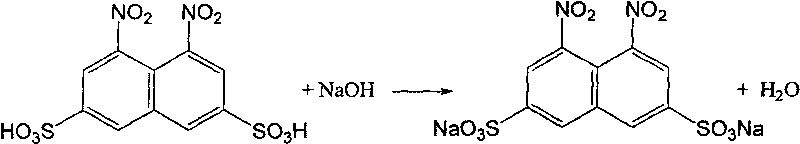Method for synthesizing dyestuff intermediate H acid by naphthalene
A technology for synthesizing dyes and intermediates, applied in 1 field, can solve problems such as difficult treatment of high-salt COD wastewater, and achieve the effects of reducing secondary pollution, reducing emissions, and reducing reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
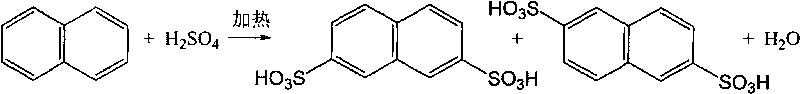
[0029] Add 192 g of refined naphthalene and 792 g of 98% sulfuric acid into a three-necked flask, raise the temperature to 160° C., stir at this temperature for 1 hour, then raise the temperature to 175° C., and stir for 6 hours to carry out the reaction. After the reaction, cool to 40°C and add 135g of water to dilute the concentration of sulfuric acid to extract refined naphthalene 2,6-disulfonic acid, heat up to 65°C and filter to obtain 142g of naphthalene 2,6-disulfonic acid, then add 130g of water to the filtrate , dilute the sulfuric acid concentration to 40%, extract naphthalene-2,7-disulfonic acid, cool to 30°C and filter to obtain 180g naphthalene-2,7-disulfonic acid.
[0030] In addition, dissolve the previously fractionated naphthalene-2,6-disulfonic acid into 50 g of 80% sulfuric acid, add 15 g of water, adjust the sulfuric acid concentration to 72%, raise the temperature to 195° C., and stir at this temperature for 10 hours for isomerization. Add the filtrate fil...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com