Cefuroxime sodium suspension powder injection
A technology of cefuroxime sodium and powder injection, which is applied in the field of a new dosage form of cefuroxime sodium, can solve problems such as poor stability, and achieve the effects of low cost, improved bioavailability and simple production process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
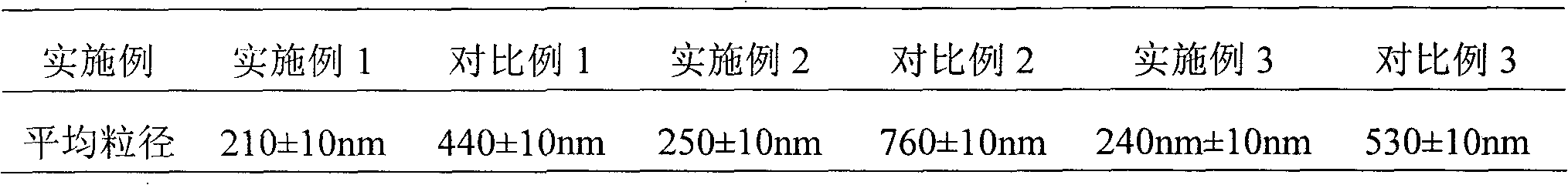
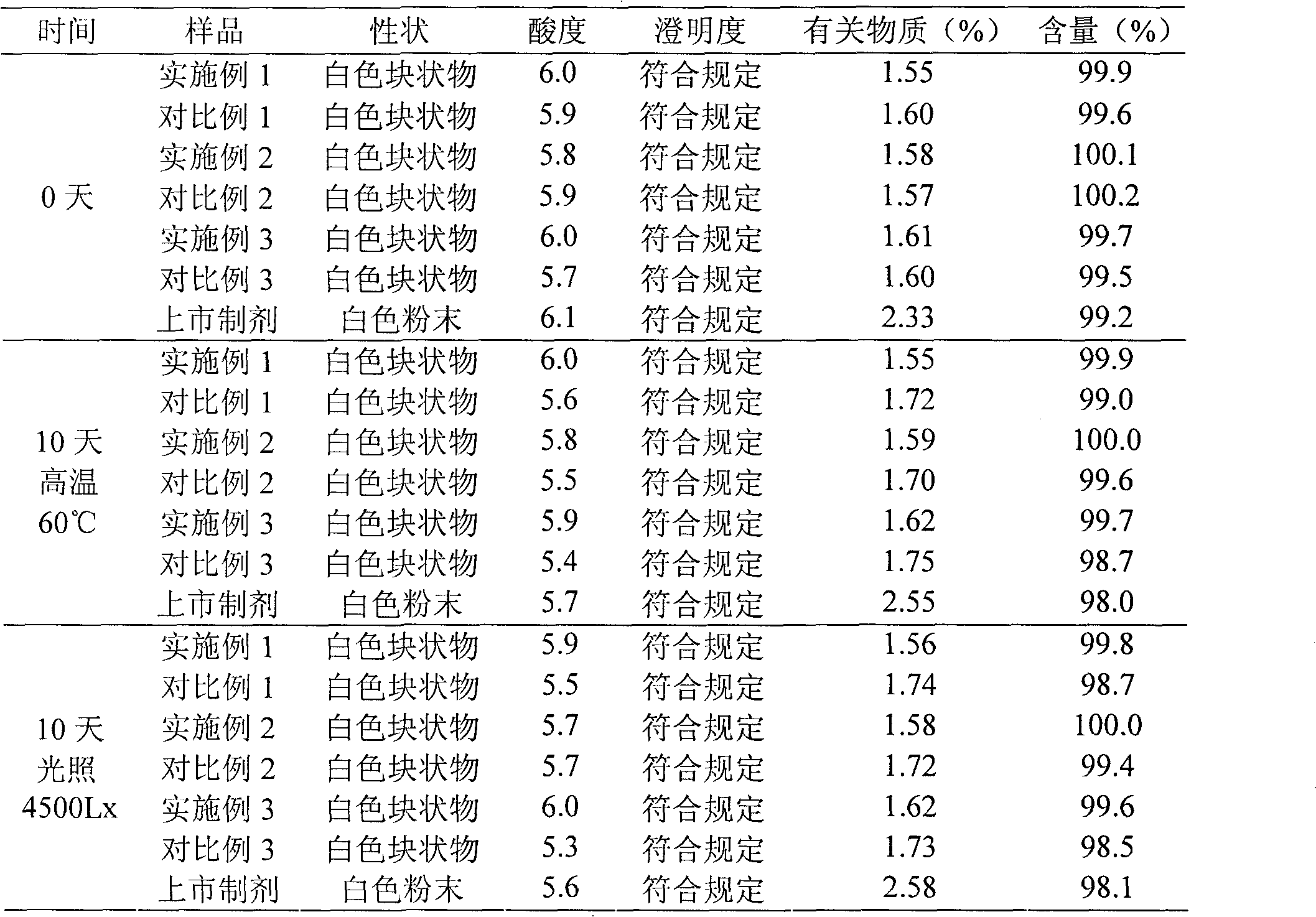
Embodiment 1
[0031] The preparation of embodiment 1 cefuroxime sodium suspension powder injection
[0032] Prescription (100 bottles): Cefuroxime Sodium 75g
[0033] Cholesterol 187.5g
[0034] Tween-80 37.5g
[0035] Mannitol 300g
[0037] Preparation Process
[0038] (1) Add 187.5g of cholesterol and 37.5g of Tween-80 into 2200ml of water for injection, then add 75g of cefuroxime sodium and mix evenly, heat and stir in a water bath at 70°C until it melts;
[0039] (2) Keeping the above liquid at 70-90°C for 10 minutes with a tissue masher to shear and stir at a speed of 10,000 r / min to obtain a primary emulsion, and then circulate and emulsify it through a high-pressure homogenizer for 5 times to obtain an emulsion;
[0040] (3) Add 300g of mannitol and 75g of sodium chloride to the emulsion, dissolve, filter, subpackage, and freeze-dry to obtain cefuroxime sodium freeze-dried suspension powder injecti...
Embodiment 2
[0048] The preparation of embodiment 2 cefuroxime sodium suspension powder injection
[0049] Prescription (100 bottles): Cefuroxime Sodium 100g
[0050] Cholesterol 500g
[0051] Tween-80 100g
[0052] Lactose 533g
[0053] Sucrose 267g
[0054] Preparation Process
[0055] (1) Add 500g of cholesterol and 100g of Tween-80 into 7500ml of water for injection, then add 100g of cefuroxime sodium and mix evenly, heat and stir in a water bath at 80°C until it melts;
[0056] (2) Heat the above liquid at 70-90°C and use a tissue masher to shear and stir for 20 minutes at a speed of 15000r / min to obtain a primary emulsion, and then circulate and emulsify it through a high-pressure homogenizer for 4 times to obtain an emulsion;
[0057] (3) Add 533g of lactose and 267g of sucrose to the emulsion, dissolve, filter, subpackage, and freeze-dry to obtain cefuroxime sodium suspension powder injection.
Embodiment 3
[0065] The preparation of embodiment 3 cefuroxime sodium suspension powder injection
[0066] Prescription (100 bottles): Cefuroxime Sodium 150g
[0067] Cholesterol 562.5g
[0068] Tween-80 112.5g
[0069] Trehalose 390g
[0070] Glycine 585g
[0071] Preparation Process
[0072] (1) Add 562.5g of cholesterol and 112.5g of Tween-80 into 6000ml of water for injection, then add 150g of cefuroxime sodium and mix evenly, heat and stir in a water bath at 90°C until it melts;
[0073] (2) Under the condition of keeping the above liquid at 70-90°C, shear and stir it with a tissue masher for 10 minutes at a speed of 13000r / min to obtain a primary emulsion, and then circulate and emulsify it through a high-pressure homogenizer for 5 times to obtain an emulsion;
[0074] (3) Add 390g trehalose and 585g glycine to the emulsion, dissolve, filter, subpackage, and freeze-dry to obtain cefuroxime sodium suspension powder injection. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com