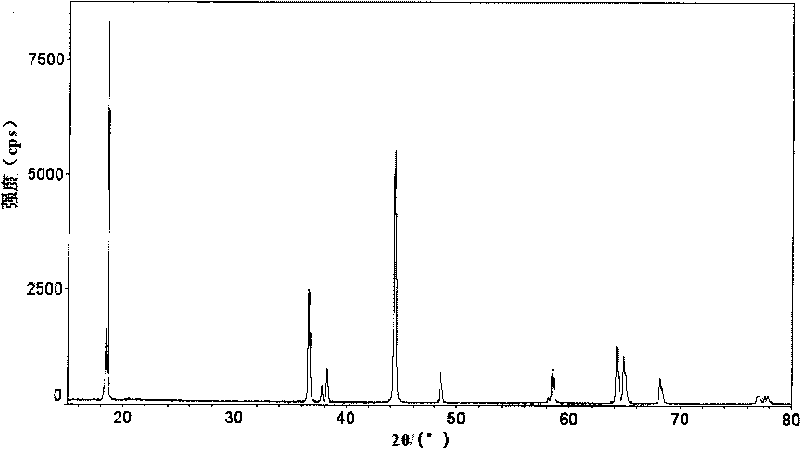
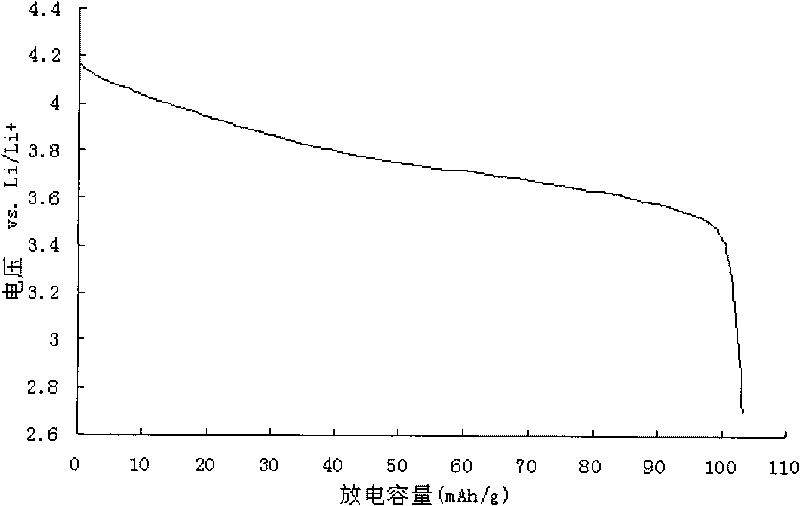
Lithium ion battery anode material and manufacturing method thereof
A lithium-ion battery, cathode material technology, applied in battery electrodes, circuits, electrical components, etc., can solve the problems affecting material cycle performance, poor thermal stability, poor structural stability, etc., to improve high-rate performance and cycle stability. The effect of improving the stability, thermal stability, and cycle performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Dissolve Ni, Co, Mn, Mg sulfate in deionized water at a ratio of 0.31:0.31:0.31:0.07 to form a solution with a total concentration of 2mol / L; 4mol / L sodium hydroxide solution, respectively, with a metering pump The salt solution and the alkali solution were evenly pumped into a stainless steel reaction tank filled with distilled water, the pH value of the reaction was controlled to be 11, the temperature was 50° C., and the reaction was carried out at a stirring speed of 1000 rad / min for 20 h. The precipitated product was filtered, washed, and dried at 300°C for 8 hours to obtain the precursor A1.
[0033] The mixture of LiOH and HF (the molar ratio of LiOH and LiF is 1.96:0.04) and the prepared precursor A 1 The molar ratio is metal lithium moles: precursor total metal moles = 1.1:1, fully mixed, and the fully mixed powder is sintered at 980 ° C for 12 hours, and ground to obtain product B 1 , B 1 The molecular formula is:
[0034] Li 1.05 [Ni 0.31 co 0.31 mn 0....
Embodiment 2
[0042] Dissolve Ni, Co, Mn, Al sulfate in the ratio of 0.31:0.31:0.31:0.07 in deionized water to form a solution with a total concentration of 2mol / L; 4mol / L sodium hydroxide solution, respectively, with a metering pump The salt solution and the alkali solution were evenly pumped into a stainless steel reaction tank filled with distilled water, the pH value of the reaction was controlled to be 11, the temperature was 50° C., and the reaction was carried out at a stirring speed of 1000 rad / min for 20 h. After the precipitated product was filtered and washed, it was dried at 300°C for 8 hours to obtain the precursor A 2 .
[0043] The mixture of LiOH and HF (the molar ratio of LiOH and LiF is 1.96:0.04) and the prepared precursor are fully mixed at a molar ratio of metal lithium moles: precursor total metal moles=1.1:1, fully The mixed powder was sintered at 980°C for 12h, and ground to obtain product B 2 , B 2 The molecular formula is:
[0044] Li 1.05 [Ni 0.31 co 0.31 m...
Embodiment 3
[0049] Dissolve Ni, Co, Mn, Cr sulfate in deionized water at a ratio of 0.31:0.31:0.31:0.07 to form a solution with a total concentration of 2mol / L; 4mol / L sodium hydroxide solution, respectively, with a metering pump The salt solution and the alkali solution were evenly pumped into a stainless steel reaction tank filled with distilled water, the pH value of the reaction was controlled to be 11, the temperature was 50° C., and the reaction was carried out at a stirring speed of 1000 rad / min for 20 h. After the precipitated product was filtered and washed, it was dried at 300°C for 8 hours to obtain the precursor A 3 .
[0050]The mixture of LiOH and HF (the molar ratio of LiOH and LiF is 1.96:0.04) and the prepared precursor are fully mixed at a molar ratio of metal lithium moles: precursor total metal moles=1.1:1, fully The mixed powder was sintered at 980°C for 12h, and ground to obtain product B 3 , B 3 The molecular formula is:
[0051] Li 1.05 [Ni 0.31 co 0.31 mn ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| First discharge capacity | aaaaa | aaaaa |
| First discharge capacity | aaaaa | aaaaa |
| First discharge capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com