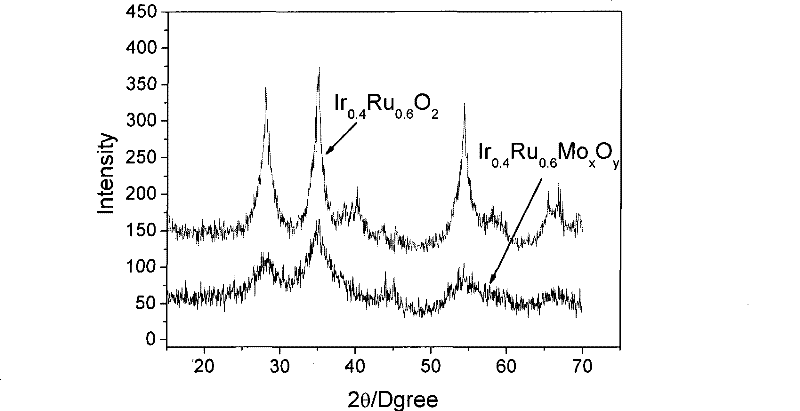
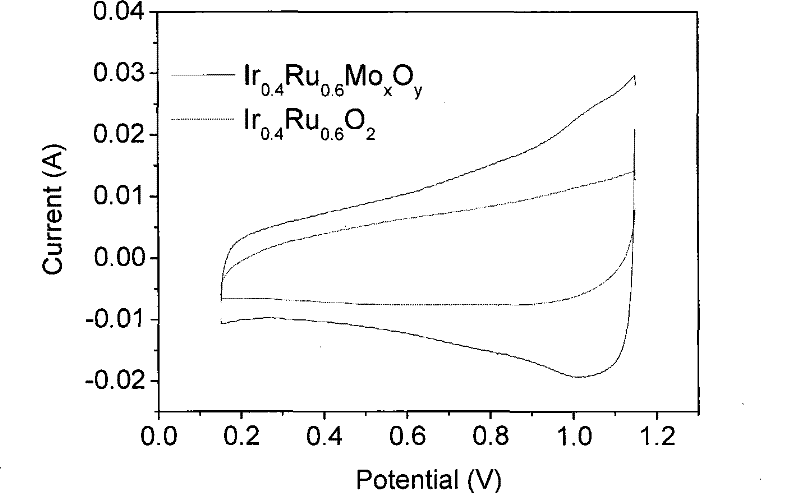
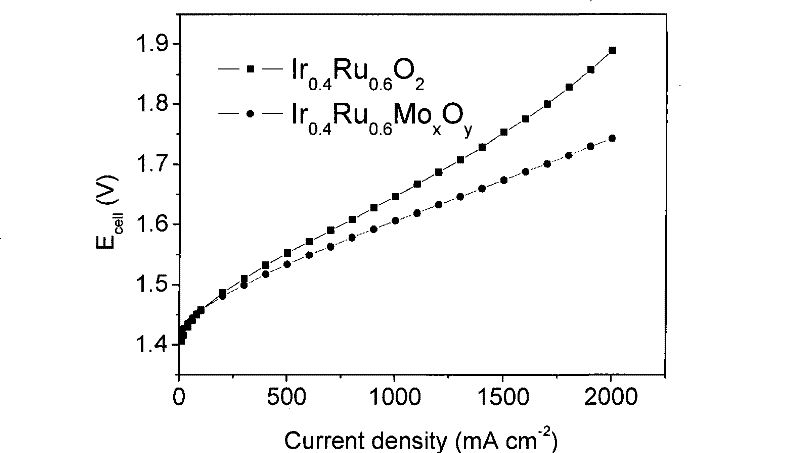
Catalyst for water electrolysis and preparation and application thereof
A water electrolysis catalyst and catalyst technology, applied in the direction of physical/chemical process catalysts, electrolysis process, electrolysis components, etc., can solve the problems of catalyst conductivity decline, etc., achieve low electrolysis voltage, low specific energy consumption, and increase specific surface area Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] 0.1617g chloroiridic acid (H 2 IrCl 6 ·6H 2 O), 0.1147g ruthenium chloride (RuCl 3 2H 2 O), 0.0943g molybdic acid (H 2 MoO 4 ·H 2 O) Dissolve in 50ml deionized water, wherein the Ir / Ru molar ratio is 2 / 3. Add 10g sodium nitrate (NaNO 3 ), stirring it to dissolve, then adding 2M NaOH aqueous solution to adjust the pH value to 7. The mixed solution was placed in a water bath at 80° C., stirred and evaporated to dryness, and then dried in an oven at 60° C. for 12 hours. The obtained solid was ground, then placed in a tube furnace, first raised to 350°C at 10°C / min, then raised to 450°C at 5°C / min, and kept at 450°C for 0.5 hours. After the product is cooled to room temperature, add deionized water to dissolve the soluble matter, then centrifuge and wash the insoluble solid, and after repeated several times, place the separated matter in a vacuum oven at 60°C for 24 hours to obtain the catalyst of the present invention Ir 0.4 Ru 0.6 Mo 0.05 o 2.15 , the mass r...
Embodiment 2
[0044] 0.167g chloroiridic acid (H 2 IrCl 6 ·6H 2 O), 0.1185g ruthenium chloride (RuCl 3 2H 2 O), 0.1443g ammonium tungstate ((NH 4 ) 5 h 5 [H 2 (WO 4 ) 6 ]·H 2 O) Dissolve in 50ml deionized water, wherein the Ir / Ru molar ratio is 2 / 3. Add 10g sodium nitrate (NaNO 3), stirring it to dissolve, then adding 2M NaOH aqueous solution to adjust the pH value to 7. The mixed solution was placed in a water bath at 80° C., stirred and evaporated to dryness, and then dried in an oven at 60° C. for 12 hours. The obtained solid was ground, then placed in a tube furnace, first raised to 350°C at 10°C / min, then raised to 450°C at 5°C / min, and kept at 450°C for 0.5 hours. After the product is cooled to room temperature, add deionized water to dissolve the soluble matter, then centrifuge and wash the insoluble solid, and after repeated several times, place the separated matter in a vacuum oven at 60°C for 24 hours to obtain the catalyst of the present invention Ir 0.4 Ru 0.6 W ...
Embodiment 3
[0049] 0.2394g chloroiridic acid (H 2 IrCl 6 ·6H 2 O), 0.1698g ruthenium chloride (RuCl 3 2H 2 O), 0.31g chromium nitrate (Cr(NO 3 ) 3 9H 2 O) Dissolve in 50ml deionized water, wherein the Ir / Ru molar ratio is 2 / 3. Add 10g sodium nitrate (NaNO 3 ), stirring it to dissolve, then adding 2M NaOH aqueous solution to adjust the pH value to 7. The mixed solution was placed in a water bath at 80° C., stirred and evaporated to dryness, and then dried in an oven at 60° C. for 12 hours. The obtained solid was ground, then placed in a tube furnace, first raised to 350°C at 10°C / min, then raised to 450°C at 5°C / min, and kept at 450°C for 0.5 hours. After the product is cooled to room temperature, add deionized water to dissolve the soluble matter, then centrifuge and wash the insoluble solid, and after repeated several times, place the separated matter in a vacuum oven at 60°C for 24 hours to obtain the catalyst of the present invention Ir 0.4 Ru 0.6 Cr 0.12 o 2.36 .
[005...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com