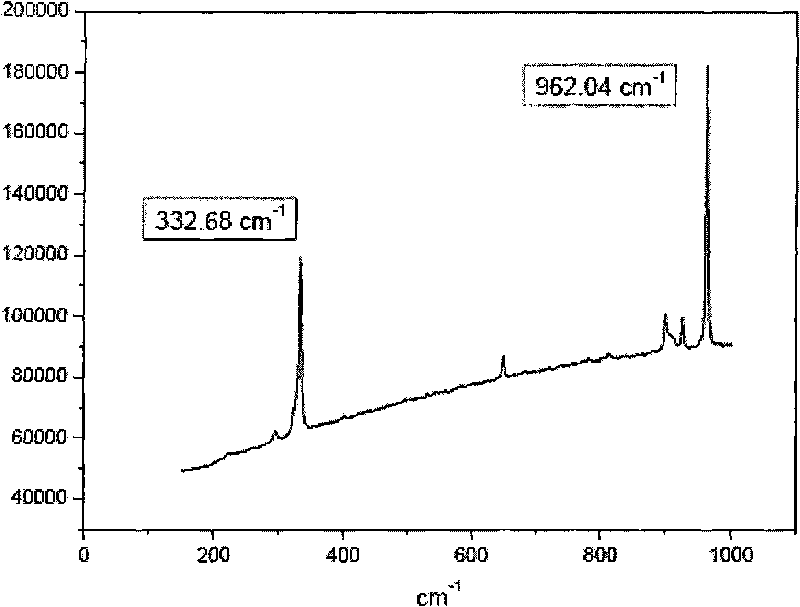
Method for preparing dissipated metal rhenium ionic liquid
A technology of ionic liquids and scattered metals, applied in chemical instruments and methods, organic compound/hydride/coordination complex catalysts, organic chemistry, etc. simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
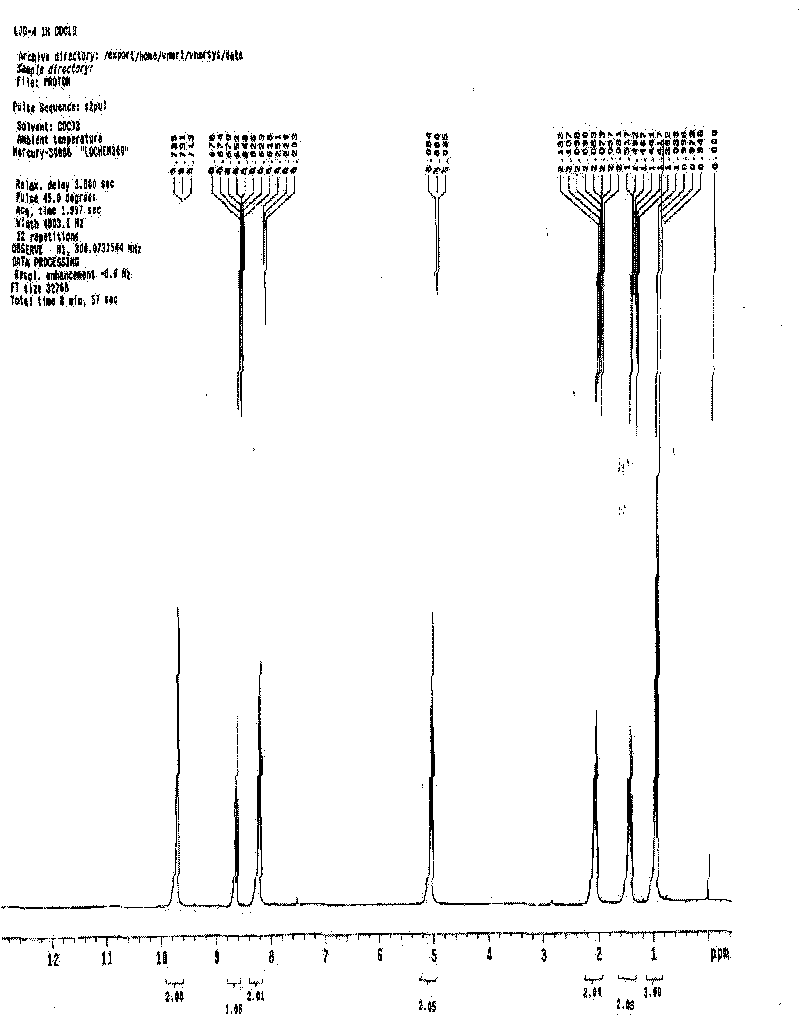
[0017] Embodiment 1N-butylpyridine perrhenate ([BPy][ReO 4 ]) ionic liquid
[0018] The preparation method is as follows:
[0019] Take a certain amount of purified N-butylpyridine, add 2 to 4 times of deionized water to dissolve it, add it to OH type anion exchange resin for ion exchange, collect the outflowing solution until bromide ion is detected. Perform an accurate titration with hydrochloric acid of known concentration to calculate the concentration of the collected solution. Add it to the ammonium perrhenate aqueous solution, the molar ratio is 1:1, heat and stir at 70°C for 4 hours, remove the water by rotary evaporation, add excess anhydrous methanol and acetonitrile solution with a volume ratio of 1:9 after cooling , sealed and vigorously stirred, placed at -34°C for 12 hours, distilled under reduced pressure to remove methanol and acetonitrile, and dried the obtained product in a vacuum oven at 80°C for 48 hours. The resulting product is N-butylpyridine perrhena...
Embodiment 2
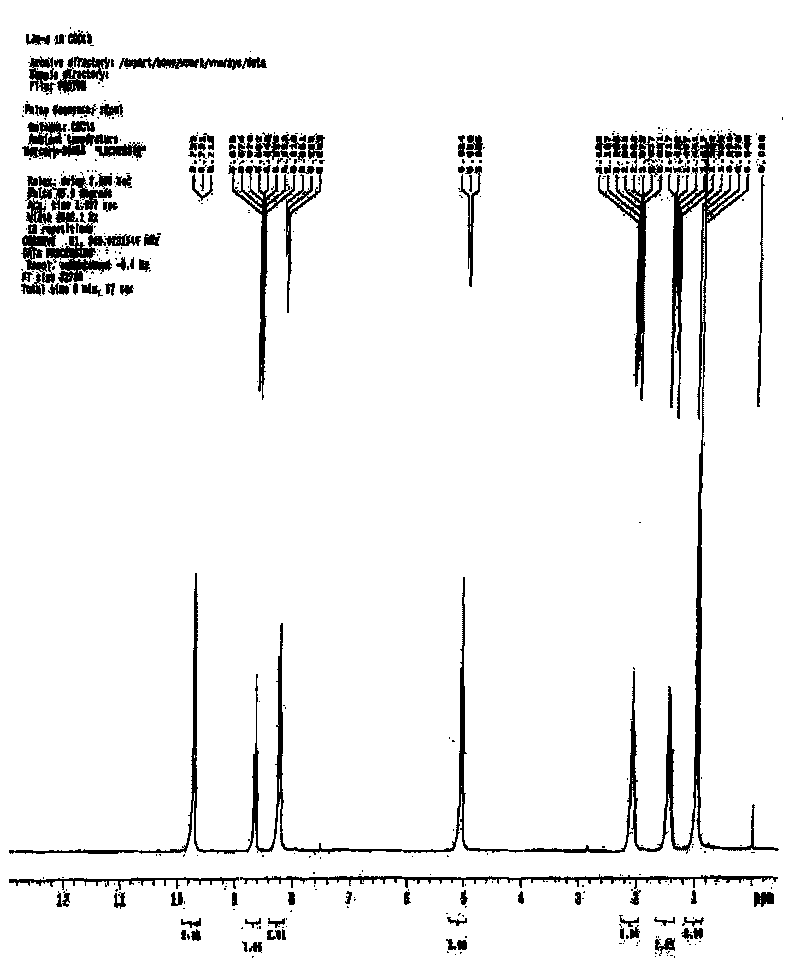
[0020] Embodiment 2N-Propylpyridine perrhenate ([TPy][ReO 4 ]) ionic liquid
[0021] The preparation method is as follows:
[0022] Take a certain amount of purified N-propylpyridine, add 2 to 4 times of deionized water to dissolve it, add it to OH type anion exchange resin for ion exchange, and collect the outflowing solution until bromide ion is detected. Perform an accurate titration with hydrochloric acid of known concentration to calculate the concentration of the collected solution. Add it to the ammonium perrhenate aqueous solution with a molar ratio of 1:2, heat and stir at 65°C for 4 hours, remove the water by rotary evaporation, and add excess anhydrous methanol and acetonitrile solution with a volume ratio of 1:9 after cooling , sealed and vigorously stirred, placed at -34°C for 12 hours, distilled under reduced pressure to remove methanol and acetonitrile, and dried the obtained product in a vacuum oven at 80°C for 48 hours. The resulting product is N-butylpyrid...
Embodiment 3
[0023] Embodiment 3N-ethylpyridine perrhenate ([EPy][ReO 4 ]) ionic liquid
[0024] The preparation method is as follows:
[0025] Take a certain amount of purified N-ethylpyridine, add 2 to 4 times of deionized water to dissolve it, add it to an OH-type anion exchange resin for ion exchange, and collect the outflowing solution until bromide ions are detected. Perform an accurate titration with hydrochloric acid of known concentration to calculate the concentration of the collected solution. Add it to the ammonium perrhenate aqueous solution, the molar ratio is 1:1.5, heat and stir at 60°C for 4 hours, remove the water by rotary evaporation, add excess anhydrous methanol and acetonitrile solution with a volume ratio of 1:9 after cooling , sealed and vigorously stirred, placed at -30°C for 12 hours, distilled under reduced pressure to remove methanol and acetonitrile, and dried the obtained product in a vacuum oven at 80°C for 48 hours. The resulting product is N-butylpyridi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com