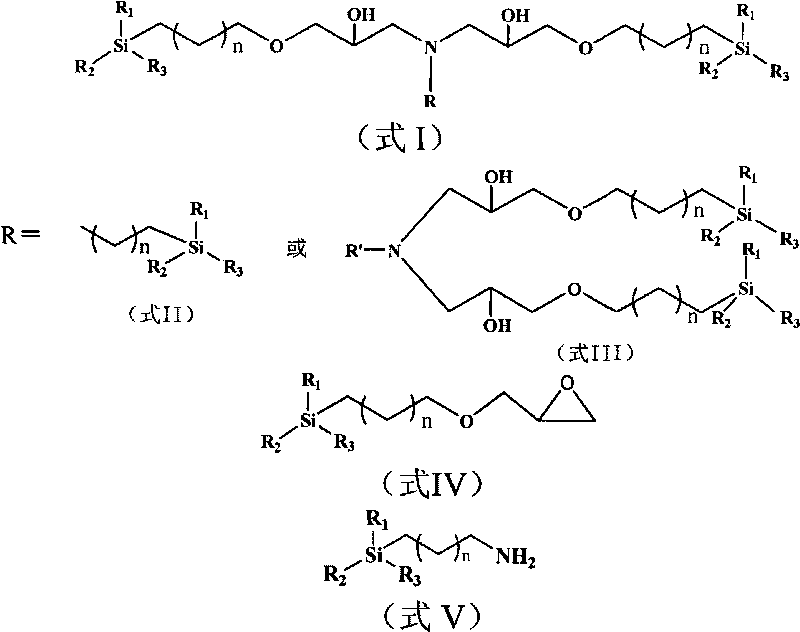
Bridged polysilsesquioxane, monomer thereof and preparation method for two
A technology of polysilsesquioxane and silane monomers is applied in the field of bridged polysilsesquioxane and its monomers and their preparation. High yield, simple synthesis method, mild process effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Under nitrogen protection, 45ml of 3-(2,3-epoxypropoxy)propyltrimethoxysilane was added to a three-necked flask equipped with a condenser, a thermometer and a dropping funnel, and then 18ml of 3-aminopropyl Trimethoxysilane, heated to 70°C, reacted for 48 hours to obtain a light yellow bridging monomer.
[0030] There are 9 trialkoxy groups in the bridging monomer obtained, take 5ml of the bridging monomer and dissolve it in 10ml of tetrahydrofuran, add 1ml of formic acid aqueous solution with a mass percentage concentration of 88%, and leave it open at 50°C for 24 hours. A bridged polysilsesquioxane is obtained.
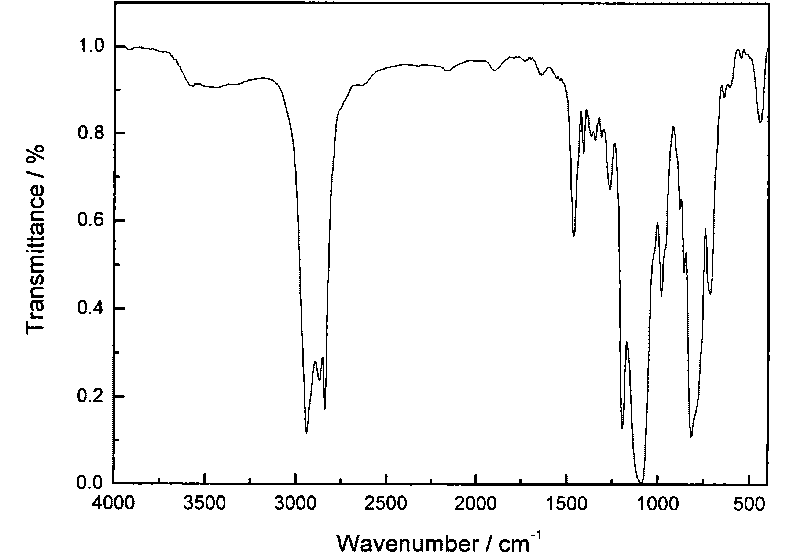
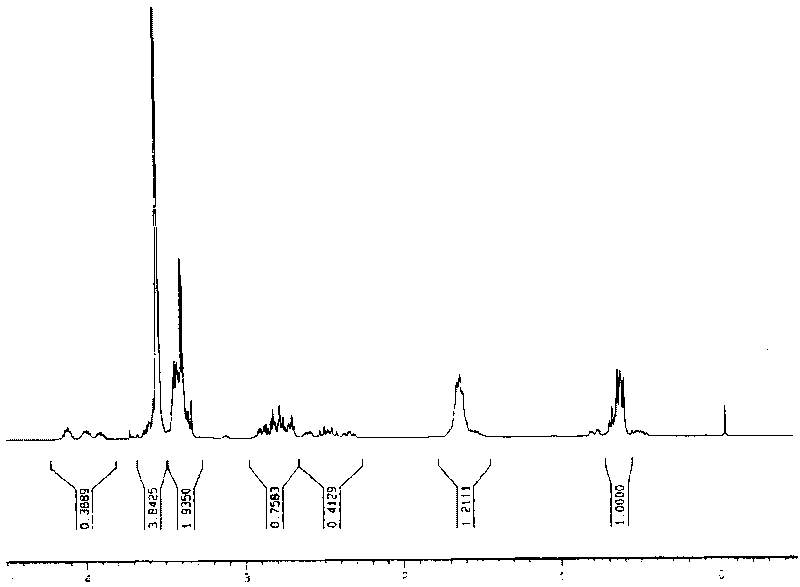
[0031] The infrared and nuclear magnetic resonance spectra of the compound are as follows figure 1 with figure 2 shown. It can be seen from the figure that the structure of the compound is correct.
Embodiment 2
[0033] Under nitrogen protection, 23ml of 3-(2,3-epoxypropoxy)propyltrimethoxysilane was added to a three-necked flask equipped with a condenser, a thermometer and a dropping funnel, and then 12ml of 3-aminopropyl Triethoxysilane, heated up to 70°C, reacted for 48 hours to obtain a light yellow bridging monomer.
[0034] There are 9 trialkoxyl groups in the bridging monomer obtained. Dissolve 5ml of the bridging monomer in 10ml of methanol, add 1ml of formic acid aqueous solution with a mass percentage concentration of 88%, and leave it open at 50°C for 24 hours. A bridged polysilsesquioxane is obtained.
Embodiment 3
[0036] Under nitrogen protection, 43ml of 3-(2,3-epoxypropoxy)propylmethyldimethoxysilane was added to a three-necked flask equipped with a condenser, a thermometer and a dropping funnel, and then 16ml of 3- Aminopropylmethyldimethoxysilane, heated to 70°C, reacted for 48 hours to obtain a light yellow bridging monomer.
[0037] There are 6 trialkoxyl groups in the bridging monomer obtained. Dissolve 5ml of the bridging monomer in 10ml of tetrahydrofuran, add 1ml of formic acid aqueous solution with a concentration of 88% by mass, and leave it open at 50°C for 36 hours. A bridged polysilsesquioxane is obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com