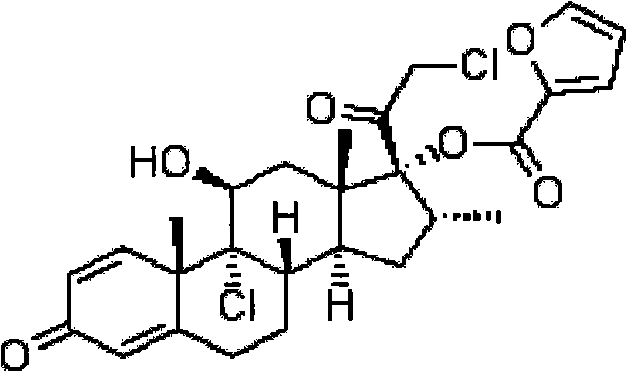
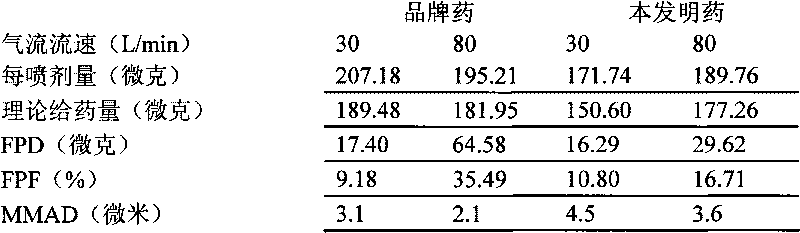
Mometasone furoate dry powder inhalation and preparing process thereof
A technology of mometasone furoate and dry powder inhaler, which can be applied in the directions of anti-inflammatory agent, respiratory system disease, non-central analgesic agent, etc., can solve problems such as complicated preparation process, and achieve simplified preparation process, convenient administration and convenience carry effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0075] Process: The active ingredient mometasone furoate is mixed with lactose 1 and lactose 2, and mixed with a high-speed shear mixer to prepare intermediate powder. In the high-speed shear mixer, two kinds of mixing blades are contained in the mixing cavity, which move in horizontal and vertical directions respectively. The dry powder mixture of the active ingredient and the pharmaceutical auxiliary material thereof is obtained. Quantitatively filling the prepared mixture into a multi-dose storage type dry powder administration device.
[0076] Element:
[0077] Mometasone Furoate 6g
[0078] Lactose 170g
[0079] Lactose II 24g
[0080] When mixing:
[0081] Stirring speed in horizontal direction 100rpm
[0082] Shear speed in vertical direction 50rpm
[0083] Mixing time 30min
Embodiment 2
[0085] Technology: with embodiment one.
[0086] Element:
[0087] Mometasone Furoate 4g
[0088] Lactose 170g
[0089] Lactose II 26g
[0090] When mixing:
[0091] Stirring speed in horizontal direction 100rpm
[0092] Vertical shear speed 300rpm
[0093] Mixing time 45min
Embodiment 3
[0095] Technology: with embodiment one.
[0096] Element:
[0097] Mometasone Furoate 6g
[0098] Lactose 1 140g
[0099] Lactose II 54g
[0100] When mixing:
[0101] Stirring speed in horizontal direction 200rpm
[0102] Vertical shear speed 300rpm
[0103] Mixing time 30min
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com