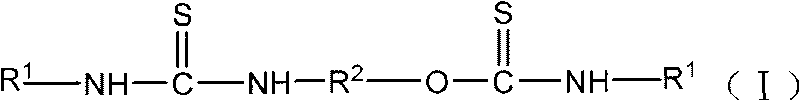
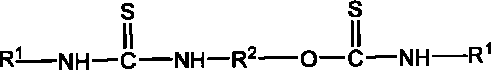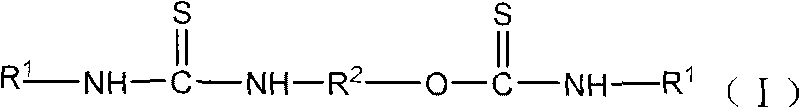Mineral flotation collectors
A collector and flotation technology, used in flotation, solid separation, etc., can solve the problems of lack of selectivity and strong collection ability, and achieve weak collection ability, high metal recovery rate, and improved flotation recovery rate. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Embodiment 1N, the preparation of N'-diethylcarboxylate-O, N "-(1,2-ethylene) thiocarbamate thiourea
[0017] 30.54 parts of monoethanolamine plus acetone are configured into a monoethanolamine-acetone solution with a mass concentration of 20%, and slowly added to the solution containing 131.2 parts of N-carboethoxyisothiocyanate at a reaction temperature of 5 to 15°C under stirring conditions. In a three-necked flask, the reaction was stirred for 2 hours, and then the reaction was continued for 4 hours at 40-50° C., and the reaction was completed. After the solvent acetone was distilled off under reduced pressure, 157.5 parts of a yellow product were obtained, which contained 145 parts of N, N'-diethylcarboxylate-O, N"-(1,2-ethylene) thiourethane thiourea, and the product purity 92.1%.
Embodiment 2
[0018] Embodiment 2N, the preparation of N'-dibenzoyl-O, N "-(1,2-ethylene) thiocarbamate thiourea
[0019] Add 30.54 parts of monoethanolamine and tetrahydrofuran to form a monoethanolamine-tetrahydrofuran solution with a mass concentration of 20%, and slowly add it to the solution containing 163.2 parts of N-benzoyl isothiocyanate at a reaction temperature of 5-20°C under stirring conditions. In a three-necked flask, stirred and reacted for 2 hours, then continued to react for 5 hours at 50-60° C., and the reaction was completed. After distilling off the solvent tetrahydrofuran under reduced pressure, 191 parts of yellow products were obtained, which contained 177 parts of N, N'-dibenzoyl-O, N"-(1,2-ethylidene) thiocarbamate thiourea. 92.8%.
[0020] (2) Application of thiourethane and thiourea complex base compounds in mineral flotation
Embodiment 3
[0021] Example 3: A copper ore ore sample, the copper minerals in the ore are mainly chalcopyrite, followed by chalcocite-blue chalcocite, tetrahedrite-arsenite and so on. Iron minerals are mainly pyrite. The raw ore contains 0.43% copper and 2.86% sulfur.
[0022] Test procedure: primary roughing; grinding fineness: -0.074mm, accounting for 68%; chemical conditions: lime dosage 1600 g / ton, pulp pH value 9.0, other chemical conditions and their results are shown in Table 1. The result of the test of table 1 shows, thiourethane thiourea composite base collector of the present invention can obtain than conventional collector butyl xanthate, Z-200 (O-isopropyl-N-ethyl thiocarbamate) and N-ethoxycarbonyl-N'-propylthiourea (monothiourea collector) has higher copper flotation recovery and better selectivity for copper-sulfur separation.
[0023] Table 1 The flotation conditions and results of thiourethane thiourea complex base compound
[0024]
[0025]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com