Method for measuring concentration of cetirizine in human urine
A cetirizine and urine drug concentration technology, applied in the field of medical testing, can solve the problems of high price, cumbersome operation, time-consuming and laborious, etc., and achieve the effect of high precision and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach
[0013] 1. Instruments and reagents:
[0014] instrument:
[0015] Waters high-performance liquid chromatography: 510 pumps, Waters486 ultraviolet detector, Maxima 820 chromatographic workstation.
[0016] Reagent:
[0017] Cetirizine hydrochloride standard substance is provided by Jiangsu Lianyungang Pharmaceutical Factory; Xiantemin (Zyrtec) coated tablet (every piece of cetirizine hydrochloride 10mg, batch number 96A26 / A) is produced by Belgian UCB company; (Decloxizine Hydrochloride) was provided by the Pharmacy Department of Changhai Hospital Affiliated to Second Military Medical University; acetonitrile was chromatographic grade 1, sodium dihydrogen phosphate, sodium hydroxide, hydrochloric acid, triethylamine, SDS, etc. were all analytically pure, and water was double distilled water.
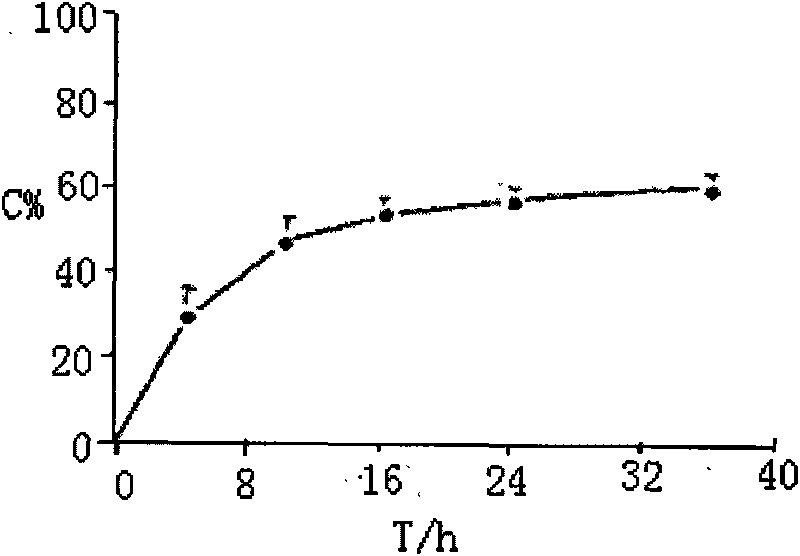
[0018] 2. Methods and Results
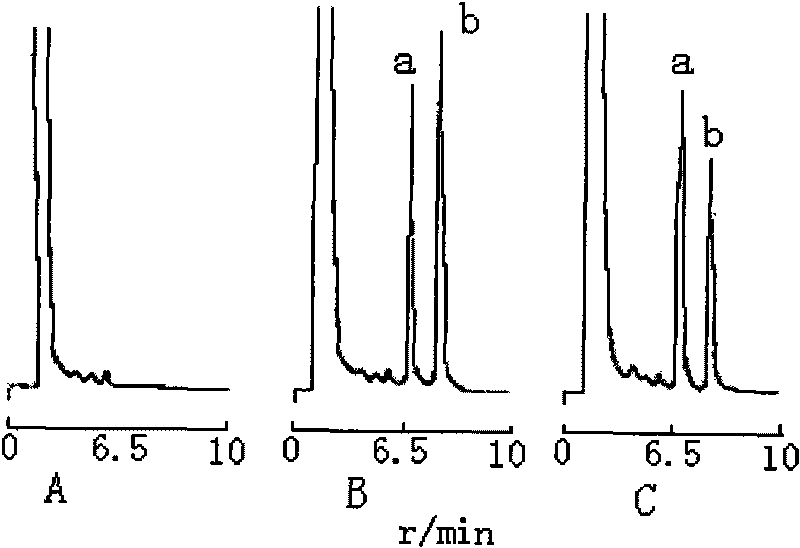
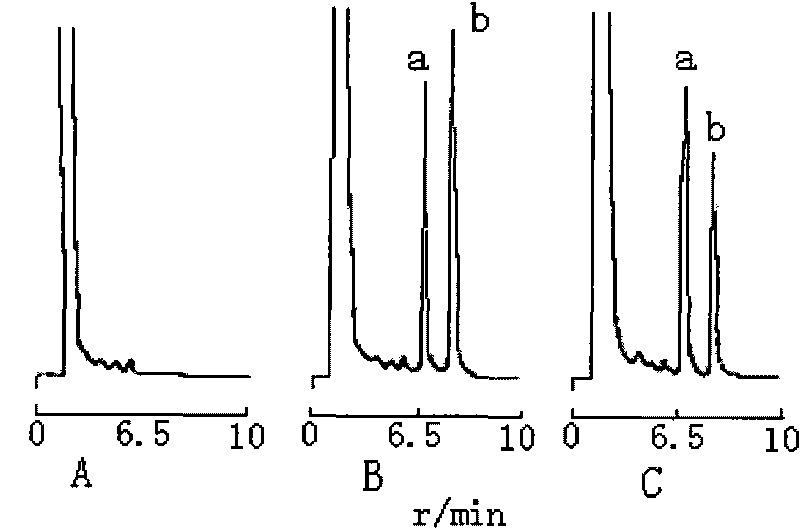
[0019] Chromatographic conditions and system suitability test: Chromatographic conditions Waters Nova-Pak C 18 (150×3.9mm, 4μm) chromatographic column;...
PUM
| Property | Measurement | Unit |
|---|---|---|
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com