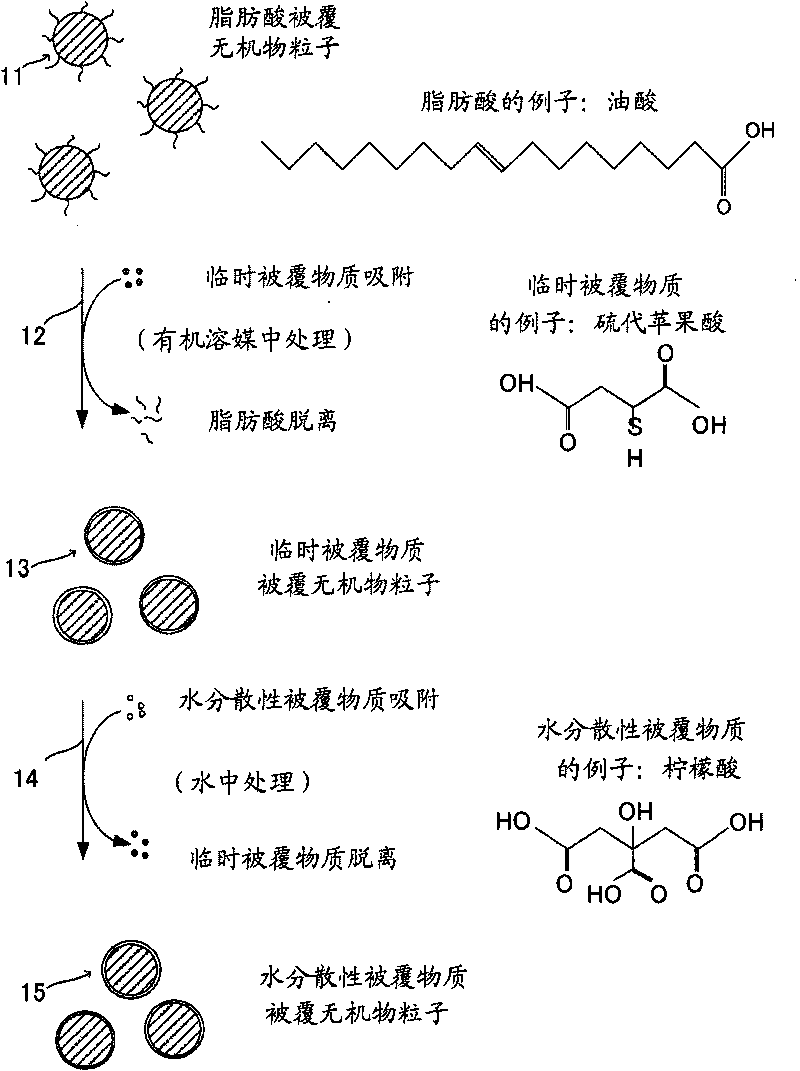
Process for production of surface-coated inorganic particles
A technology of surface coating and manufacturing method, which is applied in the field of surface coating inorganic particles, and can solve the problems of chemical instability of coated particles
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0093]
[0094] In 180 mg of an octadecene suspension of oleic acid-coated ferrite particles with an average particle size of about 8 nm and a uniform particle size, 5 ml or more of 2-propanol (manufactured by KISHIDA Chemical Co., Ltd.) was added to agglomerate the particles, and then magnetically recovered and discarded. Remove the supernatant. Furthermore, this operation was performed 3 times using 10 ml of 2-propanol. It should be noted that the octadecene suspension of oleic acid-coated ferrite particles used here is prepared by reacting ferric chloride and sodium oleate to prepare iron-oleic acid complex salt. It is obtained by dissolving in octadecene, raising the temperature to 320°C at a certain speed over 90 minutes, reacting at 320°C for 30 minutes, and then cooling to room temperature.
[0095] The 2-propanol was removed, and 16 ml of toluene was added to disperse the particles. 0.324 g of thiomalic acid (manufactured by Tokyo Chemical Industry Co., Ltd., Mw=15...
Embodiment 2
[0101]
[0102] As the temporary coating material in Example 1, 0.737 g of 1-amino-8-naphthol-3,6-sodium disulfonate (manufactured by Tokyo Chemical Industry Co., Ltd., Mw=341.29) was used. 1 The same operation was performed instead of coating the particle surface to obtain a citric acid-coated ferrite particle dispersion.
[0103] The particle size in water of the particle dispersion liquid obtained when 1-amino-8-naphthol-3,6-disulfonate sodium was used as a temporary coating material was measured by a dynamic light scattering method to obtain the size of the particles dispersed in water, Results The particle size in water was 8.0±0.9nm. The obtained particles were observed using TEM, and the average particle diameter was determined, and a value of about 8 nm was obtained. In this particle dispersion liquid, the distribution in terms of particle weight was the largest at around 8 nm, and the particles of about 8 nm in water were in a single-particle dispersed state as in ...
Embodiment 3
[0104]
[0105] As the temporary coating material in Example 1, except that 0.394 g of meso-2,3-dimercaptosuccinic acid (manufactured by Tokyo Chemical Industry Co., Ltd., Mw=182.22) was used, it was replaced by the same operation as in Example 1. The particle surface was coated to obtain a citric acid-coated ferrite particle dispersion. It should be noted that at the stage of the ferrite particle dispersion coated with the meso-2,3-dimercaptosuccinic acid as a temporary coating material, the dispersion was colored and the concentration of iron ions in the ferrite particles was observed. A part dissolves.
[0106] The particle size in water of the particle dispersion liquid obtained when meso-2,3-dimercaptosuccinic acid was used as the temporary coating material was measured by dynamic light scattering method, and the size of the particles dispersed in water was obtained. As a result, the particle size in water was 9.0±4.6nm. The obtained particles were observed using TEM,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com