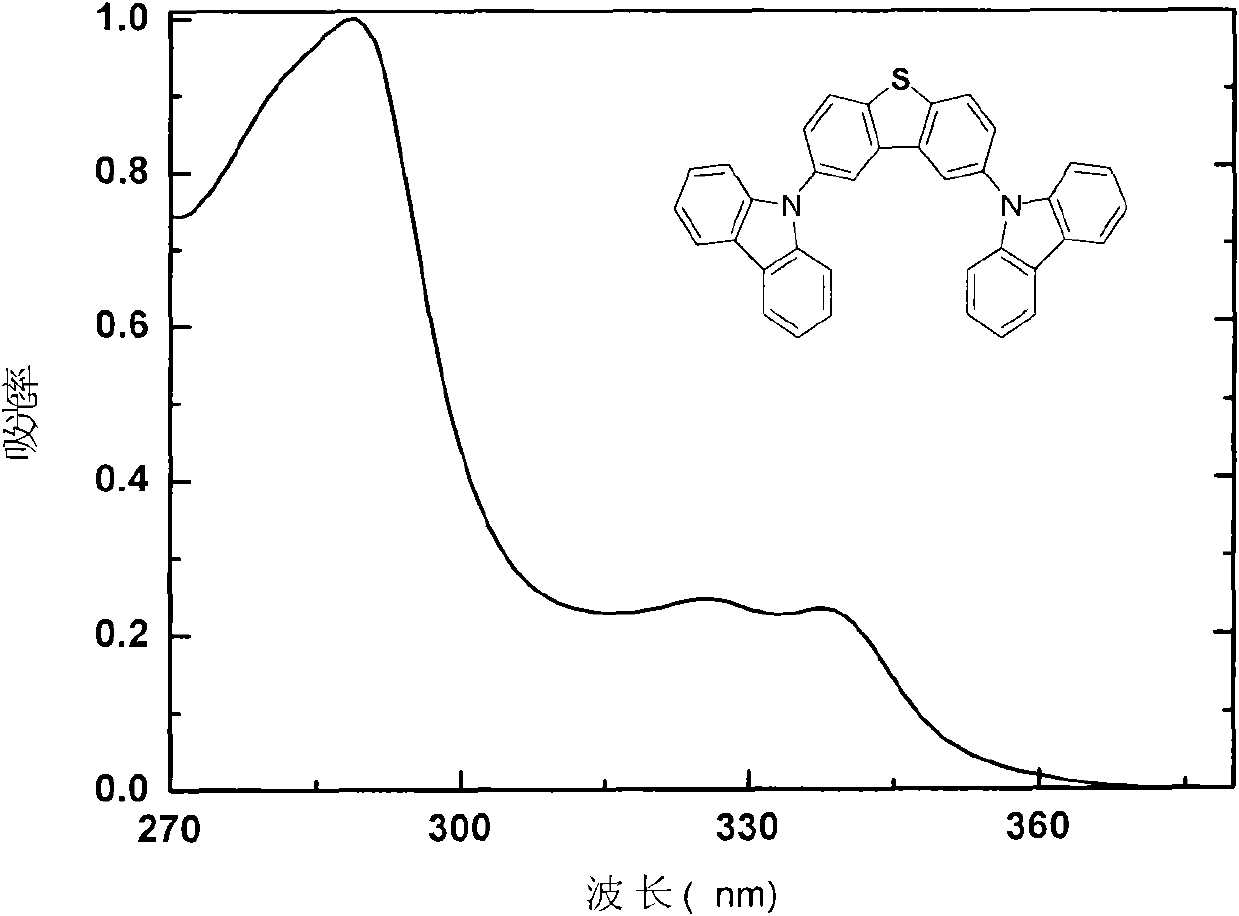
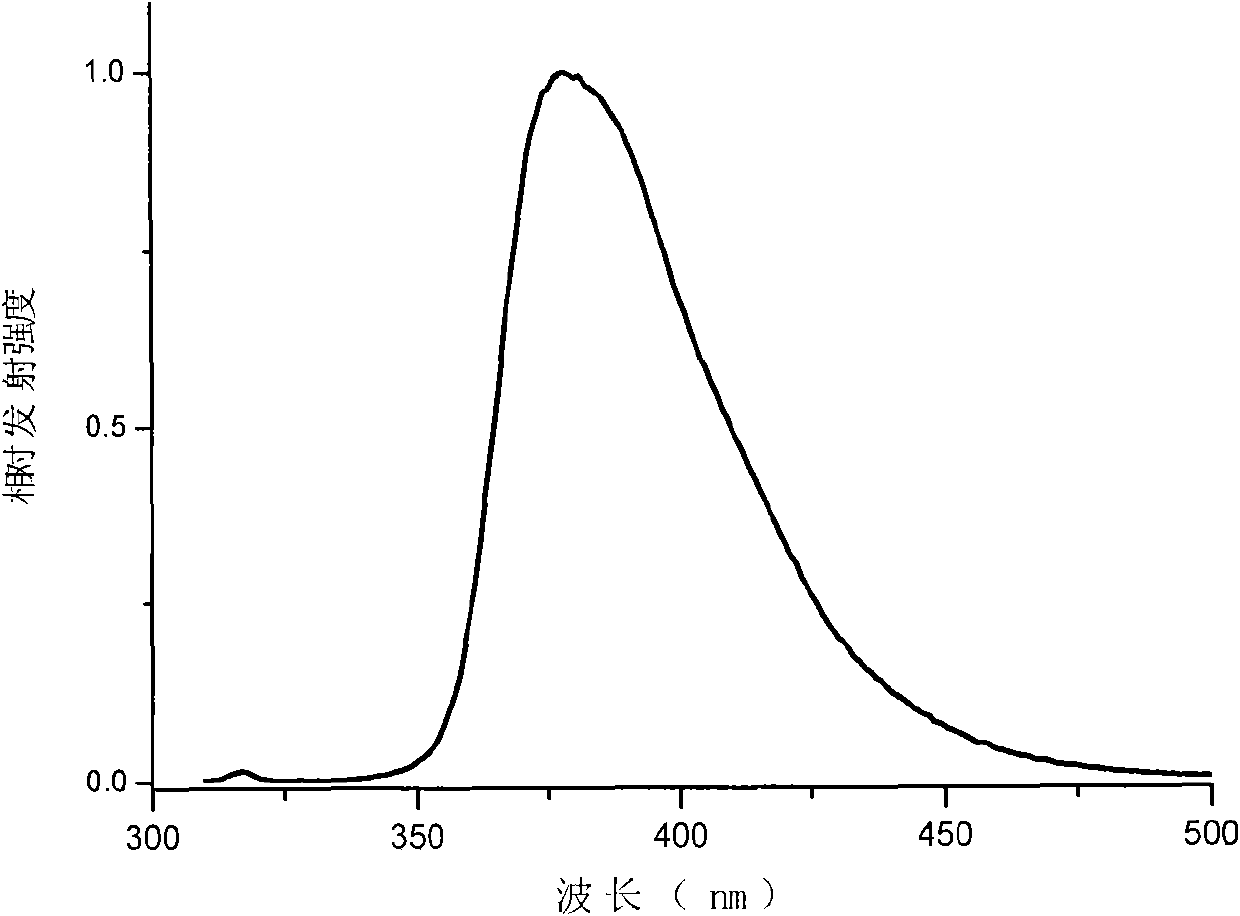
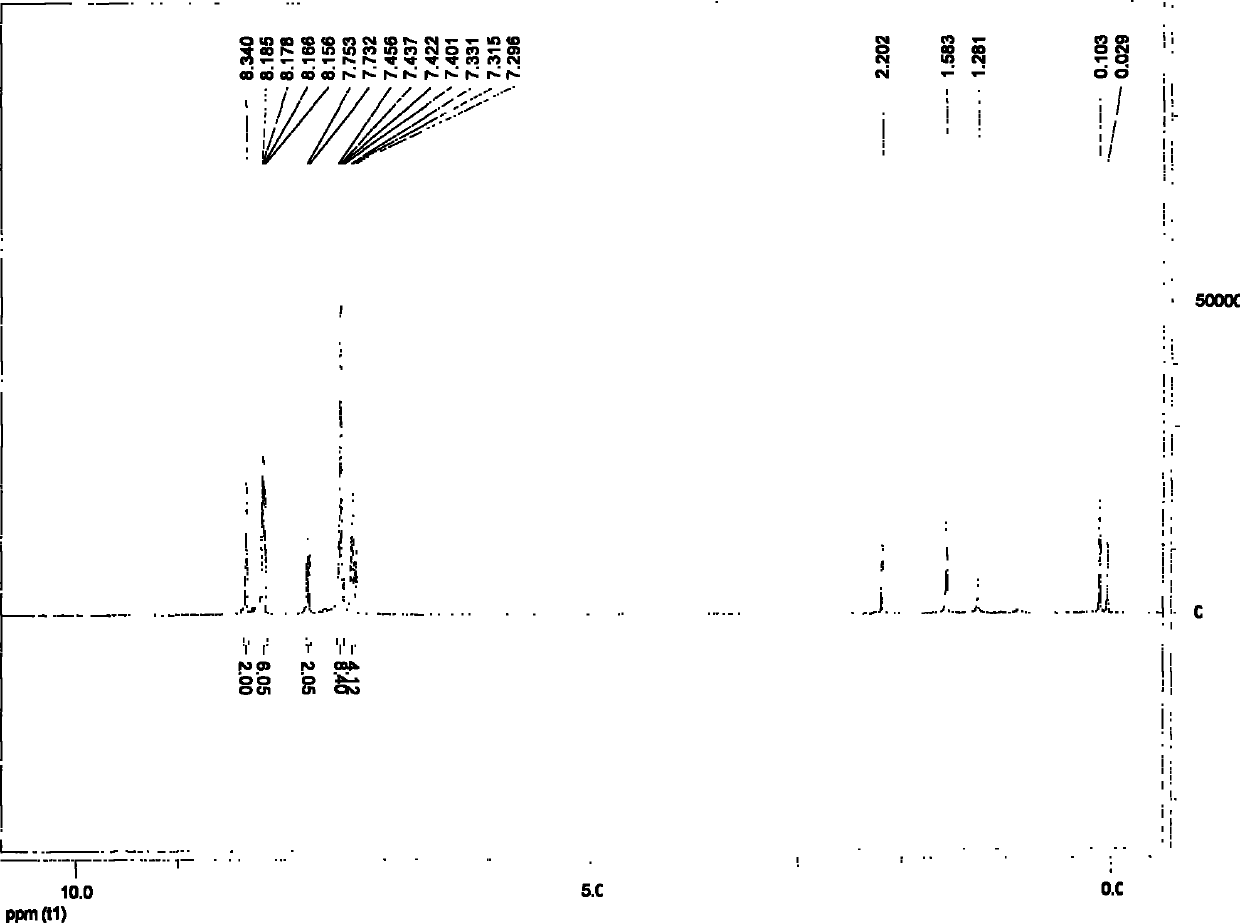
Carbazole-terminated heterofluorene main body material and preparation and application method
A main material and carbazole-encapsulated technology, which is applied in chemical instruments and methods, luminescent materials, semiconductor/solid-state device manufacturing, etc., can solve problems such as no patent reports, achieve good thermal stability and solubility, and high reaction yield , the effect of high triplet energy levels
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Synthesis of Carbazole-terminated Thiofene
[0041] Add carbazole, 3,6-dibromothiofluorene, electrolytic copper powder, and anhydrous potassium carbonate into a two-necked flask equipped with a stirring bar. After the flask is dried and sealed, it is evacuated three times and ventilated with nitrogen. Before using high-purity nitrogen, It must be processed and used in strict anhydrous and oxygen-free devices. Then, put the reaction device into the oil bath, and add nitrobenzene with a syringe. The nitrobenzene must be strictly treated before use to remove some impurities. The whole reaction system was reacted for 24h under reflux. After the reaction was completed, nitrobenzene was distilled off under reduced pressure, washed with deionized water and extracted three times with dichloromethane, and the organic phase was spin-dried through a silica gel column to obtain a white solid.
Embodiment 2
[0043] Synthesis of carbazole-capped carbazole
[0044]Add carbazole, N-octyl-3,6-dibromocarbazole, electrolytic copper powder, and anhydrous potassium carbonate into a two-necked flask equipped with a stirring bar, and after the flask is dried and airtight, evacuate and pass nitrogen three times , High-purity nitrogen must be treated in strict anhydrous and oxygen-free devices before use. Then, put the reaction device into the oil bath, and add nitrobenzene with a syringe. The nitrobenzene must be strictly treated before use to remove some impurities. The whole reaction system was reacted for 24h under reflux. After the reaction was completed, nitrobenzene was distilled off under reduced pressure, washed with deionized water and extracted three times with dichloromethane, and the organic phase was spin-dried through a silica gel column to obtain a white solid.
Embodiment 3
[0046] Synthesis of Carbazole-terminated Phosphorene
[0047] Add 4,4'-dibromo-6,6'-diiodo-3,3'-dimethoxybiphenyl into a single-necked flask equipped with a stirring bar, and after the flask is dried and sealed, evacuate three times and pass nitrogen gas , High-purity nitrogen must be treated in strict anhydrous and oxygen-free devices before use. Then, place the reaction device in ether and a dry ice bath, cool it to -100°C and add butyl lithium to react at this temperature for half an hour, then add dichlorophenylphosphine, react overnight at room temperature, and use dichloro Methane extraction and column chromatography gave white solid 2,7-dibromo-3,6-dimethoxy-9,phenyl-phosphorene.
[0048] Add carbazole, 2,7-dibromophosphorene, electrolytic copper powder, and anhydrous potassium carbonate into a two-necked flask equipped with a stirring bar. After the flask is dried and sealed, it is evacuated three times and ventilated with nitrogen. Before using high-purity nitrogen, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com